Deck 6: Properties of Gases: the Air We Breathe
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/106
Play
Full screen (f)
Deck 6: Properties of Gases: the Air We Breathe
1
Which of the following graphs shows the correct relationship between the temperature and volume of an ideal gas at a given pressure? Note the origin corresponds to V = 0 and T = 0.
A)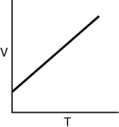
B)
C)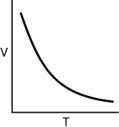
D)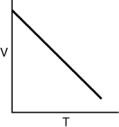
A)

B)

C)

D)


2
A barometer measures a pressure of 745 mm Hg. What is this pressure in atm?
A) 0.980 atm
B) 1.02 atm
C) 1.03 * 105 atm
D) 1.00 * 104 atm
E) 0.556 atm
A) 0.980 atm
B) 1.02 atm
C) 1.03 * 105 atm
D) 1.00 * 104 atm
E) 0.556 atm
0.980 atm
3
What is the volume of a gas that exerts a pressure of 457 mm Hg if it exerted a pressure of 2.50 atm when its volume was 25.0 mL?
A) 9.62 mL
B) 1.80 L
C) 0.104 L
D) 6.01 mL
E) 25.0 L
A) 9.62 mL
B) 1.80 L
C) 0.104 L
D) 6.01 mL
E) 25.0 L
0.104 L
4
What force is exerted by exactly one atmosphere of pressure (1.013* 105 Pa) on a mercury barometer with an inner diameter of 20.00 mm? Recall that the area of a circle is A = r2 and that 1 N = 1 kg m/s2.
A) 31.82 N
B) 15.91 N
C) 127.3 N
D) 3.182 * 107 N
E) 40.52 N
A) 31.82 N
B) 15.91 N
C) 127.3 N
D) 3.182 * 107 N
E) 40.52 N

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
5
The atmospheric pressure is reported to be 31.5 in of mercury. The prevailing weather system on this day would be classified as __________
A) a cold front.
B) a warm front.
C) low pressure.
D) high pressure.
E) normal.
A) a cold front.
B) a warm front.
C) low pressure.
D) high pressure.
E) normal.

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
6
If the pressure in the eye of a hurricane is 645 mbar, what is the corresponding pressure in mm Hg? Recall 1.013 bar = 1 atm.
A) 637 mm Hg
B) 0.637 mm Hg
C) 484 mm Hg
D) 497 mm Hg
E) 645 mm Hg
A) 637 mm Hg
B) 0.637 mm Hg
C) 484 mm Hg
D) 497 mm Hg
E) 645 mm Hg

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
7
Which of the following graphs shows the correct relationship between the pressure and temperature of an ideal gas in a given volume? Note the origin corresponds to P = 0 and T = 0.
A)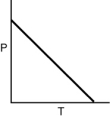
B)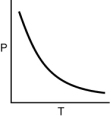
C)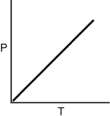
D)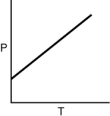
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
8
What height of water (in meters) is necessary to measure a pressure of 760 mm Hg? The density of mercury is 13.79 g/cm3, and the density of water is 1.00 g/cm3.
A) 0.760 m
B) 1.05 * 104 m
C) 0.105 m
D) 10.5 m
E) 55.1 mm
A) 0.760 m
B) 1.05 * 104 m
C) 0.105 m
D) 10.5 m
E) 55.1 mm

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
9
The pressure of a gas is inversely proportional to __________
A) the volume of the gas.
B) the number of gas particles.
C) the temperature of the gas.
D) the molar mass of the gas.
E) the mass of the gas.
A) the volume of the gas.
B) the number of gas particles.
C) the temperature of the gas.
D) the molar mass of the gas.
E) the mass of the gas.

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
10
Which of the following substances will require the highest column for measuring an atmospheric pressure of 0.950 atm? (Assume all the barometers have columns with the same diameter.)
A) Mercury (d = 13.59 g/cm3)
B) Ethanol (d = 0.79 g/cm3)
C) Ethylene glycol (d = 1.09 g/cm3)
D) Water (d = 1.00 g/cm3)
E) Carbon tetrachloride (d = 1.58 g/cm3)
A) Mercury (d = 13.59 g/cm3)
B) Ethanol (d = 0.79 g/cm3)
C) Ethylene glycol (d = 1.09 g/cm3)
D) Water (d = 1.00 g/cm3)
E) Carbon tetrachloride (d = 1.58 g/cm3)

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
11
What pressure (in Pa) will be exerted on the bottom of a 63.5 kg hiker's foot when she stands on a rusty nail? Assume the diameter of the tip of the nail is 1.00 mm. The acceleration due to gravity is 9.81 m/s2. Recall that the area of a circle is: A = r2 and that 1 Pa = 1 kg m-1 s-2.
A) 1.98 * 102 Pa
B) 7.93 * 102 Pa
C) 1.98 * 108 Pa
D) 7.93 *108 Pa
E) 7.93 * 105 Pa
A) 1.98 * 102 Pa
B) 7.93 * 102 Pa
C) 1.98 * 108 Pa
D) 7.93 *108 Pa
E) 7.93 * 105 Pa

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
12
What pressure will be measured (in Pa) if a gas is exerting a force of 10.00 N on a mercury barometer with an inner diameter of 10.00 mm? Recall that the area of a circle is A = r2 and that 1 N = 1 kg m/s2.
A) 3.183 * 104 Pa
B) 1.273 *105 Pa
C) 3.183* 10-2 Pa
D) 1.273*10-2 Pa
A) 3.183 * 104 Pa
B) 1.273 *105 Pa
C) 3.183* 10-2 Pa
D) 1.273*10-2 Pa

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
13
A balloon is filled with 3.00 L of helium at a pressure of 765 torr. What is the volume of the balloon at an altitude where the pressure in the balloon is 530 torr?
A) 0.231 L
B) 4.33 L
C) 2.08 L
D) 1.00 L
E) 3.00 L
A) 0.231 L
B) 4.33 L
C) 2.08 L
D) 1.00 L
E) 3.00 L

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
14
A sample of gas at 4.0 atm and 25.0 mL is heated from 25°C to 40°C. If the pressure remains constant, what is the final volume of the gas?
A) 26.2 mL
B) 40.0 mL
C) 23.8 mL
D) 105 mL
E) 25.0 mL
A) 26.2 mL
B) 40.0 mL
C) 23.8 mL
D) 105 mL
E) 25.0 mL

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
15
The atmospheric pressure in the eye of a hurricane is found to be 26.6 in of mercury. The pressure in the eye of this hurricane is __________
A) 760 mm Hg.
B) 1.00 *105 Pa.
C) 0.889 atm.
D) 0.987 bar.
E) 0.785 atm.
A) 760 mm Hg.
B) 1.00 *105 Pa.
C) 0.889 atm.
D) 0.987 bar.
E) 0.785 atm.

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
16
Two identical cement blocks are placed on a glass table in different positions as shown in the diagram. Which statement about these blocks is correct? 
A) A exerts more pressure on the table.
B) B exerts more pressure on the table.
C) Both blocks exert the same pressure on the table.
D) A exerts more force on the table.
E) B exerts more force on the table.

A) A exerts more pressure on the table.
B) B exerts more pressure on the table.
C) Both blocks exert the same pressure on the table.
D) A exerts more force on the table.
E) B exerts more force on the table.

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
17
Which of the following graphs shows the correct relationship between pressure and volume for n moles of gas at temperature T?
A)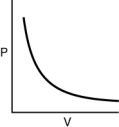
B)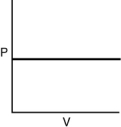
C)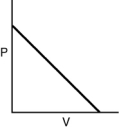
D)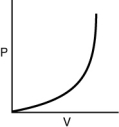
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
18
A gas, initially at 1.00 atm and 5.00 L, is compressed to a pressure of 15.00 atm. What is the volume of the gas at this pressure?
A) 3.00 L
B) 333 mL
C) 0.333 mL
D) 3.00 mL
E) 5.00 L
A) 3.00 L
B) 333 mL
C) 0.333 mL
D) 3.00 mL
E) 5.00 L

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
19
What is the pressure in the gas bulb connected to the mercury manometer shown in the diagram if the ambient pressure is 750 torr? The heights labeled in the diagram are 15 mm and 21 mm. 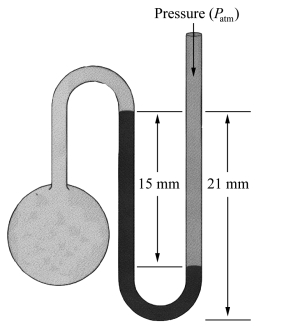
A) 756 torr
B) 771 torr
C) 729 torr
D) 765 torr
E) 735 torr

A) 756 torr
B) 771 torr
C) 729 torr
D) 765 torr
E) 735 torr

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
20
The cold inflation pressure of many car tires is 30 lb/in2, which is the gauge pressure. If 1 atm = 14.7 lb/in2, what is the gauge pressure of a car tire in torr?
A) 2.04 torr
B) 1.55*103 torr
C) 441 torr
D) 0.580 torr
E) 1.12* 104 torr
A) 2.04 torr
B) 1.55*103 torr
C) 441 torr
D) 0.580 torr
E) 1.12* 104 torr

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
21
Which one of these samples contains the smallest number of molecules?
A) 1.0 L of H2 at STP (0°C and 1atm)
B) 1.0 L of N2 at STP
C) 1.0 L of H2 at 20°C and 760 torr
D) 1.0 L of N2 at 0°C and 800 torr
E) 1.0 L of He at STP
A) 1.0 L of H2 at STP (0°C and 1atm)
B) 1.0 L of N2 at STP
C) 1.0 L of H2 at 20°C and 760 torr
D) 1.0 L of N2 at 0°C and 800 torr
E) 1.0 L of He at STP

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
22
Which of the following samples of an ideal gas has the lowest density?
A)
B)
C)
D)
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
23
Calcium carbonate (100.1 g/mol) decomposes according to the following reaction equation: CaCO3(s) CaO(s) + CO2(g)
What pressure of CO2 gas will form in a 25.0 mL jar containing 5.00 g CaCO3 at 300 K if 0.00500% of the solid decomposes?
A) 0.246 atm
B) 2.46 atm
C) 1.00 atm
D) 2.46 *103 atm
E) 2.46 * 10-3 atm
What pressure of CO2 gas will form in a 25.0 mL jar containing 5.00 g CaCO3 at 300 K if 0.00500% of the solid decomposes?
A) 0.246 atm
B) 2.46 atm
C) 1.00 atm
D) 2.46 *103 atm
E) 2.46 * 10-3 atm

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
24
Which of the gases shown here will exert the lowest pressure, assuming that each container has the same volume and temperature?
A)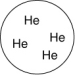
B)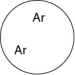
C)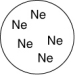
D)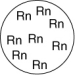
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
25
The density of a pure gas at STP depends on its __________
A) molar mass.
B) concentration.
C) temperature.
D) volume.
E) pressure.
A) molar mass.
B) concentration.
C) temperature.
D) volume.
E) pressure.

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
26
A 1.50 mol sample of He occupies a volume of 2.50 L at a pressure of 14.7 atm. What will be the pressure of a 1.50 mol sample of H2 gas under the same conditions?
A) 7.33 atm
B) 14.7 atm
C) 29.4 atm
D) 1.00 atm
E) 24.5 atm
A) 7.33 atm
B) 14.7 atm
C) 29.4 atm
D) 1.00 atm
E) 24.5 atm

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
27
In an experiment, 7.5 mol of a gas are held in a 3.5 L container at 39°C. What pressure does the gas exert if it is assumed to be ideal?
A) 5.5 * 103 atm
B) 2.9 * 104 atm
C) 6.9 atm
D) 55 atm
E) 96 atm
A) 5.5 * 103 atm
B) 2.9 * 104 atm
C) 6.9 atm
D) 55 atm
E) 96 atm

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
28
What is the pressure of a 3.5 mmol sample of ethane (C2H6) gas contained in a 0.500 L flask at 298 K?
A) 171 mm Hg
B) 1.30 *105 mm Hg
C) 130 mm Hg
D) 326 mm Hg
E) 17.1 mm Hg
A) 171 mm Hg
B) 1.30 *105 mm Hg
C) 130 mm Hg
D) 326 mm Hg
E) 17.1 mm Hg

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
29
Which of the following reactions will result in a reduced total pressure?
A) CH4(g) + 2O2(g) CO2(g) + 2H2O(g)
B) 2N2O(g) 2N2(g) + O2(g)
C) 2HI(g) H2(g) + I2(g)
D) 2H2(g) + O2(g) 2H2O( )
)
A) CH4(g) + 2O2(g) CO2(g) + 2H2O(g)
B) 2N2O(g) 2N2(g) + O2(g)
C) 2HI(g) H2(g) + I2(g)
D) 2H2(g) + O2(g) 2H2O(
 )
)
Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
30
What is the temperature if 5.0 g of methane (CH4) in a 100.0 mL container exerts a pressure of 42 atm?
A) -110°C
B) +164°C
C) -270°C
D) -263°C
E) +263°C
A) -110°C
B) +164°C
C) -270°C
D) -263°C
E) +263°C

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
31
What volume is occupied by 1.00 mol of an ideal gas at 1.00 atm and 0.00°C?
A) 22.4 L
B) 22.7 L
C) 22.1 L
D) 15.0 L
E) 27.2 L
A) 22.4 L
B) 22.7 L
C) 22.1 L
D) 15.0 L
E) 27.2 L

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
32
An unknown gas held in a 25.0 mL flask at 45°F exerts a pressure of 25 mtorr. By using this information with the ideal gas law, it is possible to determine __________
A) the mass of the gas in the container.
B) the identity of the gas in the container.
C) the number of molecules of the gas in the container.
D) the molecular geometry of the gas in the container.
E) nothing more about the gas.
A) the mass of the gas in the container.
B) the identity of the gas in the container.
C) the number of molecules of the gas in the container.
D) the molecular geometry of the gas in the container.
E) nothing more about the gas.

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
33
How many moles of propane are contained in a 5.00 L tank at 450 torr and 25°C?
A) 0.12 mol
B) 92 mol
C) 1.4 mol
D) 1100 mol
E) 41 mol
A) 0.12 mol
B) 92 mol
C) 1.4 mol
D) 1100 mol
E) 41 mol

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
34
The pressure gauge on a cylinder used to fill balloons with He shows a pressure of 1275 psi at a temperature of 25°C. Assuming the cylinder has a volume of 10 L, how many grams of He does the cylinder contain?
A) 520 g
B) 142 g
C) 6.70 * 10 -4 g
D) 37.0 g
E) 4.00 g
A) 520 g
B) 142 g
C) 6.70 * 10 -4 g
D) 37.0 g
E) 4.00 g

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
35
Indicate the gas with the greatest density at STP.
A) CH4
B) He
C) Ar
D) H2
E) O2
A) CH4
B) He
C) Ar
D) H2
E) O2

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
36
In an experiment, 25.0 mL of a gas with a pressure of 1.00 atm is contained in a balloon at 25.0°C. The balloon is then cooled to 5.0°C, and the pressure is found to be 0.750 atm. What is the volume of the gas under the new conditions?
A) 17.5 mL
B) 20.1 mL
C) 25.0 mL
D) 31.1 mL
E) 35.7 mL
A) 17.5 mL
B) 20.1 mL
C) 25.0 mL
D) 31.1 mL
E) 35.7 mL

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
37
What volume is occupied by 1.00 mol of an ideal gas at 1.00 bar and 0.00°C?
A) 22.4 L
B) 22.7 L
C) 22.1 L
D) 15.0 L
E) 27.2 L
A) 22.4 L
B) 22.7 L
C) 22.1 L
D) 15.0 L
E) 27.2 L

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
38
Which of the following is unimportant when using the ideal gas law?
A) The chemical identity of the gas sample.
B) The temperature of the gas sample.
C) The pressure of the gas sample.
D) The volume of the container holding the gas sample.
E) The amount of gas.
A) The chemical identity of the gas sample.
B) The temperature of the gas sample.
C) The pressure of the gas sample.
D) The volume of the container holding the gas sample.
E) The amount of gas.

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
39
Which of the gas samples shown below in identical containers has the lowest density (g/L)?
A)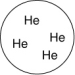
B)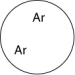
C)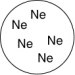
D)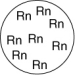
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
40
Four containers, each with the same volume and at the same temperature, are shown in the following diagrams. Which container is at the highest pressure?
A)
B)
C)
D)
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
41
What is the density of propane gas (CH3CH2CH3, 44.1 g/mol) at 25°C and 650 mm Hg?
A) 1.54 g/L
B) 3.55 g/mL
C) 18.3 g/L
D) 2.05 g/L
E) 1.17 g/L
A) 1.54 g/L
B) 3.55 g/mL
C) 18.3 g/L
D) 2.05 g/L
E) 1.17 g/L

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
42
The density of a gaseous compound of nitrogen and oxygen is 4.11 g/L at STP. What is the most likely formula of this gas?
A) N2O4
B) N2O5
C) N2O
D) NO2
E) NO
A) N2O4
B) N2O5
C) N2O
D) NO2
E) NO

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
43
In a mixture of hydrogen and helium gases, the mole fraction of helium is 0.750. If the partial pressure of hydrogen in the mixture is 75 torr, what is the total pressure of the mixture?
A) 100 torr
B) 300 torr
C) 19 torr
D) 750 torr
E) 760 torr
A) 100 torr
B) 300 torr
C) 19 torr
D) 750 torr
E) 760 torr

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
44
Which container shown below has the largest partial pressure of helium? The white circles represent helium atoms; the dark circles represent argon atoms.
A)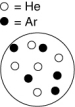
B)
C)
D)
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
45
Which container shown below has the largest partial pressure of argon? The white circles represent helium atoms; the dark circles represent argon atoms.
A)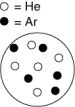
B)
C)
D)
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
46
The density of an unknown gas is 1.72 g/L at 750.0 torr and 30.0°C. Which of the following could be the unknown gas?
A) CS2
B) NO
C) N2O
D) NH3
E) NO2
A) CS2
B) NO
C) N2O
D) NH3
E) NO2

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
47
What is the molar mass of a gas that has a density of 2.62 g/L at 2.00 atm and 25.0°C?
A) 32.0 g/mol
B) 16.0 g/mol
C) 64.0 g/mol
D) 28.0 g/mol
E) 2.69 g/mol
A) 32.0 g/mol
B) 16.0 g/mol
C) 64.0 g/mol
D) 28.0 g/mol
E) 2.69 g/mol

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
48
In which of the following containers is the mole fraction of helium greatest? The white circles represent helium atoms; the dark circles represent argon atoms.
A)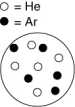
B)
C)
D)
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
49
During the decomposition of CaCO3(s) into CaO(s) and O2(g), 100.0 mL of gas is collected by the displacement of water in a flask at 25°C with an atmospheric pressure of 755 mm Hg. The vapor pressure of water at this temperature is 23.8 mm Hg. What mass of oxygen was collected?
A) 0.679 mg
B) 21.7 mg
C) 12.9 mg
D) 0.807 mg
E) 126 mg
A) 0.679 mg
B) 21.7 mg
C) 12.9 mg
D) 0.807 mg
E) 126 mg

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
50
The partial pressure of a gas is __________
A) the pressure the gas exerts when pure.
B) the same as the vapor pressure of the gas.
C) the pressure due to the gas in a mixture.
D) the total pressure of a mixture of gases.
E) the pressure exerted by one molecule of the gas.
A) the pressure the gas exerts when pure.
B) the same as the vapor pressure of the gas.
C) the pressure due to the gas in a mixture.
D) the total pressure of a mixture of gases.
E) the pressure exerted by one molecule of the gas.

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
51
What is the mole fraction of H2 in a mixture with a total pressure of 800 torr when the partial pressure of H2 is 90 torr?
A) 0.11
B) 8.8
C) 1.0
D) 0.5
E) 0.88
A) 0.11
B) 8.8
C) 1.0
D) 0.5
E) 0.88

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
52
Nitrous oxide (N2O, also known as laughing gas) has been used as an anesthetic. What is the density of N2O at 750 torr and 30°C?
A) 1.2 g/L
B) 1.1 g/L
C) 1.8 g/L
D) 1.7 g/L
E) 1.5 g/L
A) 1.2 g/L
B) 1.1 g/L
C) 1.8 g/L
D) 1.7 g/L
E) 1.5 g/L

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
53
In which of the following containers is the mole fraction of argon greatest? The white circles represent helium atoms; the dark circles represent argon atoms.
A)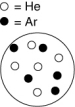
B)
C)
D)
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
54
N2O (laughing gas) is used as an anesthetic. A dentist has a 2.00 L flask of N2O at a pressure of 1.00 atm and a 3.00 L flask of nitrogen gas at 2.00 atm that are separated by a valve. What is the pressure in the flasks when the valve is opened, and the gases mix together? Both flasks are at the same temperature, and the temperature does not change when the gases mix. 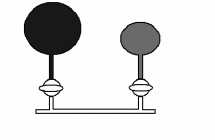
A) 3.00 atm
B) 1.50 atm
C) 1.60 atm
D) 1.20 atm
E) 1.00 atm

A) 3.00 atm
B) 1.50 atm
C) 1.60 atm
D) 1.20 atm
E) 1.00 atm

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
55
Which of the following gases has nearly the same density as CO2 at room temperature and atmospheric pressure?
A) N2
B) O2
C) N2O
D) Ar
E) CO
A) N2
B) O2
C) N2O
D) Ar
E) CO

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
56
A mixture of cyclopropane gas (C3H6) and oxygen (O2) in a 1.00 to 4.00 mole ratio is used as an anesthetic. What mass of cyclopropane gas is present in a 2.00 L flask at 23.5°C if the total pressure of the oxygen and propane is 1.00 atm? (The molar mass of C3H6 is 42.078 g/mol.)
A) 0.69 g
B) 3.45 g
C) 2.76 g
D) 6.83 mg
E) 8.73 g
A) 0.69 g
B) 3.45 g
C) 2.76 g
D) 6.83 mg
E) 8.73 g

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
57
Which of the containers shown below has the lowest total pressure? The white circles represent helium atoms; the dark circles represent argon atoms.
A)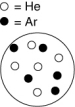
B)
C)
D)
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
58
Which of the following graphs shows how the density of an ideal gas changes with temperature?
A)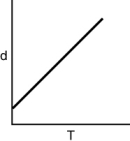
B)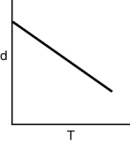
C)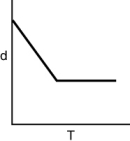
D)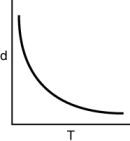
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
59
A gas mixture has partial pressures of 120 torr N2, 350 torr Ar, and 480 torr SF6. What is the mole fraction of sulfur hexafluoride in the mixture?
A) 0.13
B) 0.37
C) 0.45
D) 0.51
E) 0.55
A) 0.13
B) 0.37
C) 0.45
D) 0.51
E) 0.55

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
60
Which one of the following gases could most easily be differentiated from propane (CH3CH2CH3) on the basis of density when compared at the same temperature and pressure?
A) CO2
B) N2O
C) NO2
D) F2
E) CH2
 CH-CH3
CH-CH3
A) CO2
B) N2O
C) NO2
D) F2
E) CH2
 CH-CH3
CH-CH3
Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
61
What is the temperature of a sample of ozone (O3) with an average kinetic energy of 1.73 kJ/mol?
A) 10 K
B) 270 K
C) 139 K
D) 280 K
E) 79 K
A) 10 K
B) 270 K
C) 139 K
D) 280 K
E) 79 K

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
62
What is the root-mean-square speed of N2O at 25°C?
A) 13.0 m/s
B) 411 m/s
C) 127 m/s
D) 37.9 m/s
E) 18.6 m/s
A) 13.0 m/s
B) 411 m/s
C) 127 m/s
D) 37.9 m/s
E) 18.6 m/s

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
63
What is the average kinetic energy (in kJ/mol) of nitrous oxide (N2O) molecules at 30.0°C?
A) 0.906 kJ/mol
B) 3.78 kJ/mol
C) 288 kJ/mol
D) 147 kJ/mol
E) 2.52 kJ/mol
A) 0.906 kJ/mol
B) 3.78 kJ/mol
C) 288 kJ/mol
D) 147 kJ/mol
E) 2.52 kJ/mol

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
64
Which of these gases (Ar, N2O, H2) has the same average kinetic energy at 25°C as CO2?
A) Ar
B) N2O
C) H2
D) They are all the same as CO2.
E) They are all different from CO2.
A) Ar
B) N2O
C) H2
D) They are all the same as CO2.
E) They are all different from CO2.

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
65
Determine the root-mean-square speed of methane (CH4) molecules that have an average kinetic energy of 10.5 J/mol.
A) 337 m/s
B) 1.14 m/s
C) 2.81 * 1013 m/s
D) 36.2 m/s
E) 1150 m/s
A) 337 m/s
B) 1.14 m/s
C) 2.81 * 1013 m/s
D) 36.2 m/s
E) 1150 m/s

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
66
_____ is how gases escape through a hole in a container, and _____ is how gases spread among each other.
A) Diffusion; effusion
B) Effusion; diffusion
C) Effusion; effusion
D) Diffusion; diffusion
A) Diffusion; effusion
B) Effusion; diffusion
C) Effusion; effusion
D) Diffusion; diffusion

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
67
Which set of gases is listed from slowest to fastest diffusion rate?
A) HCN < H2 < He < O3
B) H2 < He < HCN < O3
C) O3 < HCN < He < H2
D) HCN < He < O3 < H2
E) H2 < HCN < He < O3
A) HCN < H2 < He < O3
B) H2 < He < HCN < O3
C) O3 < HCN < He < H2
D) HCN < He < O3 < H2
E) H2 < HCN < He < O3

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
68
Helium-neon (HeNe) lasers contain a low-pressure mixture of He and Ne gases. How much greater is the root-mean-square speed of He atoms than Ne atoms in a HeNe laser?
A) 5.0
B) 0.20
C) 2.2
D) 1.6
E) 0.45
A) 5.0
B) 0.20
C) 2.2
D) 1.6
E) 0.45

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
69
_____ is how gases spread among each other; and _____ is how gases escape through a hole in a container.
A) Diffusion; effusion
B) Effusion; diffusion
C) Effusion; effusion
D) Diffusion; diffusion
A) Diffusion; effusion
B) Effusion; diffusion
C) Effusion; effusion
D) Diffusion; diffusion

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
70
Which set of gases is listed from fastest to slowest diffusion rate?
A) HCN > H2 > He > O3
B) H2 > He > HCN > O3
C) O3 > HCN > He > H2
D) HCN > He > O3 > H2
E) H2 > HCN > He > O3
A) HCN > H2 > He > O3
B) H2 > He > HCN > O3
C) O3 > HCN > He > H2
D) HCN > He > O3 > H2
E) H2 > HCN > He > O3

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
71
Which set of gases is listed from slowest to fastest effusion rate?
A) N2O < NO2 < NO < N2O4
B) NO < N2O < NO2 < N2O4
C) N2O4 < NO2 < N2O < NO
D) NO < NO2 < N2O < N2O4
E) N2O4 < N2O < NO2 < NO
A) N2O < NO2 < NO < N2O4
B) NO < N2O < NO2 < N2O4
C) N2O4 < NO2 < N2O < NO
D) NO < NO2 < N2O < N2O4
E) N2O4 < N2O < NO2 < NO

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
72
Which set of gases is listed from fastest to slowest effusion rate?
A) N2O > NO2 > NO > N2O4
B) NO > N2O > NO2 > N2O4
C) N2O4 > NO2 > N2O > NO
D) NO > NO2 > N2O > N2O4
E) N2O4 > N2O > NO2 > NO
A) N2O > NO2 > NO > N2O4
B) NO > N2O > NO2 > N2O4
C) N2O4 > NO2 > N2O > NO
D) NO > NO2 > N2O > N2O4
E) N2O4 > N2O > NO2 > NO

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
73
Which of the following gases will effuse through a hole in a balloon fastest?
A) Kr
B) NO
C) N2O
D) NO2
E) Ar
A) Kr
B) NO
C) N2O
D) NO2
E) Ar

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
74
Which set of gases is listed from lowest to highest root-mean-square speed at 25°C?
A) Kr < CO2 < NO2 < SF6
B) SF6 < NO2 < CO2 < Kr
C) SF6 < Kr < CO2 < NO2
D) SF6 < Kr < NO2 < CO2
E) CO2 < NO2 < Kr < SF6
A) Kr < CO2 < NO2 < SF6
B) SF6 < NO2 < CO2 < Kr
C) SF6 < Kr < CO2 < NO2
D) SF6 < Kr < NO2 < CO2
E) CO2 < NO2 < Kr < SF6

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
75
The root-mean-square speed of a gas is found to be 391.2 m/s at 270 K. Which of the following is the gas?
A) N2
B) O2
C) Ar
D) CO2
E) CO
A) N2
B) O2
C) Ar
D) CO2
E) CO

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
76
Which one of the following statements about the kinetic molecular theory is not correct?
A) The size of a molecule is negligible.
B) Gas molecules move constantly and randomly throughout the space they occupy.
C) The average kinetic energy of a gas molecule is directly proportional to the absolute temperature of the gas.
D) Collisions of gas molecules with each other and with the walls of the container are elastic.
E) Gas molecules attract and repel each other.
A) The size of a molecule is negligible.
B) Gas molecules move constantly and randomly throughout the space they occupy.
C) The average kinetic energy of a gas molecule is directly proportional to the absolute temperature of the gas.
D) Collisions of gas molecules with each other and with the walls of the container are elastic.
E) Gas molecules attract and repel each other.

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
77
Which set of gases is listed from highest to lowest root-mean-square speed at 25°C?
A) Kr > CO2 > NO2 > SF6
B) SF6 > NO2 > CO2 > Kr
C) SF6 > Kr > CO2 > NO2
D) SF6 > Kr > NO2 > CO2
E) CO2 > NO2 > Kr > SF6
A) Kr > CO2 > NO2 > SF6
B) SF6 > NO2 > CO2 > Kr
C) SF6 > Kr > CO2 > NO2
D) SF6 > Kr > NO2 > CO2
E) CO2 > NO2 > Kr > SF6

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
78
The diffusion rate of H2 in a lecture hall was found to be 25.0 m/sec at 25°C. How long will it take poisonous HCN gas to diffuse 10 m in the same room?
A) 1.5 s
B) 0.11 s
C) 5.4 s
D) 0.19 s
E) 2.7 s
A) 1.5 s
B) 0.11 s
C) 5.4 s
D) 0.19 s
E) 2.7 s

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
79
Which of the following figures is the most accurate representation of a gas sample? The arrows show the velocities of individual molecules.
A)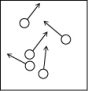
B)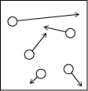
C)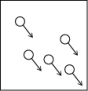
D)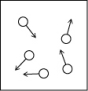
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
80
Which one of the following statements is not correct? In a sample of air at a given temperature ____________
A) the nitrogen and oxygen molecules have the same average kinetic energy.
B) the nitrogen and oxygen molecules have the same average speed.
C) some nitrogen molecules are moving slower than some oxygen molecules.
D) some nitrogen molecules are moving faster than some oxygen molecules.
E) all the molecules are moving.
A) the nitrogen and oxygen molecules have the same average kinetic energy.
B) the nitrogen and oxygen molecules have the same average speed.
C) some nitrogen molecules are moving slower than some oxygen molecules.
D) some nitrogen molecules are moving faster than some oxygen molecules.
E) all the molecules are moving.

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck



