Deck 5: Chemical Reactions
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/89
Play
Full screen (f)
Deck 5: Chemical Reactions
1
Aspartic acid is an amino acid used to synthesize proteins. What is the molar mass of aspartic acid shown below? 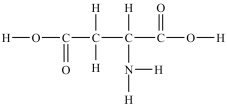
A)121.09 g/mol
B)133.11 g/mol
C)117.0 g/mol
D)126.04 g/mol
E)132.09 g/mol

A)121.09 g/mol
B)133.11 g/mol
C)117.0 g/mol
D)126.04 g/mol
E)132.09 g/mol
B
2
Suppose the theoretical yield in a reaction is 10.5 g and the percent yield is 75.5%. What is the actual yield of product obtained?
A)793 g
B)7.93 g
C)13.9 g
D)0.139 g
A)793 g
B)7.93 g
C)13.9 g
D)0.139 g
B
3
How many moles of carbon dioxide are in 211 g of carbon dioxide?
A)929 mol of CO2
B)4.79 mol of CO2
C)167 mol of CO2
D)0.209 mol of CO2
A)929 mol of CO2
B)4.79 mol of CO2
C)167 mol of CO2
D)0.209 mol of CO2
B
4
Write a balanced chemical equation for the reaction of acetone (C3H6O)with oxygen (O2)to form carbon dioxide (CO2)and water (H2O).
A)C3H6O + O2 CO2 + H2O
B)C3H6O + 4 O2 3 CO2 + 3 H2O
C)3 CO2 + 3 H2O C3H6O + 4 O2
D)6 C3H6O + 8 O2 6 CO2 + 6 H2O
A)C3H6O + O2 CO2 + H2O
B)C3H6O + 4 O2 3 CO2 + 3 H2O
C)3 CO2 + 3 H2O C3H6O + 4 O2
D)6 C3H6O + 8 O2 6 CO2 + 6 H2O

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
5
Consider the oxidation of sodium metal to sodium oxide described by the balanced equation: 4 Na + O2 2 Na2O. What is the theoretical yield of Na2O in grams from 9.0 mol of O2?
A)11 g of Na2O
B)410 g of Na2O
C)1, 100 g of Na2O
D)280 g of Na2O
E)560 g of Na2O
A)11 g of Na2O
B)410 g of Na2O
C)1, 100 g of Na2O
D)280 g of Na2O
E)560 g of Na2O

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
6
The law of conservation of mass states that
A)atoms cannot be created or destroyed in a chemical reaction.
B)molecules cannot be created or destroyed in a chemical reaction.
C)compounds cannot be created or destroyed in a chemical reaction.
D)heat cannot be created or destroyed in a chemical reaction.
A)atoms cannot be created or destroyed in a chemical reaction.
B)molecules cannot be created or destroyed in a chemical reaction.
C)compounds cannot be created or destroyed in a chemical reaction.
D)heat cannot be created or destroyed in a chemical reaction.

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
7
What is the formula weight of KCl?
A)74.55 amu
B)66.42 amu
C)36.00 amu
D)1386 amu
A)74.55 amu
B)66.42 amu
C)36.00 amu
D)1386 amu

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
8
In the chemical equation 2 Co(NO3)3 + 3 (NH4)2S Co2S3 + 6 NH4NO3 , how many nitrogen atoms are on each side of the equation?
A)2
B)3
C)6
D)12
E)16
A)2
B)3
C)6
D)12
E)16

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
9
Which sample contains the largest number of molecules?
A)100 g of CO2
B)100 g of CH4
C)100 g of CBr4
D)100 g of CHBr3
A)100 g of CO2
B)100 g of CH4
C)100 g of CBr4
D)100 g of CHBr3

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
10
How many moles of sulfur trioxide are formed from 3 moles of oxygen using the given balanced equation? 2 SO2 + O2 2 SO3
A)1 mol of SO3
B)2 mol of SO3
C)3 mol of SO3
D)5 mol of SO3
E)6 mol of SO3
A)1 mol of SO3
B)2 mol of SO3
C)3 mol of SO3
D)5 mol of SO3
E)6 mol of SO3

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
11
What is the mass of 3.81 mol of PH3?
A)34.0 g
B)3.81 g
C)8.92 g
D)130. g
E)0.112 g
A)34.0 g
B)3.81 g
C)8.92 g
D)130. g
E)0.112 g

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
12
Consider the reaction: 2 Al(OH)3 + 3 H2SO4 Al2(SO4)3 + 6 H2O. How many grams of Al2(SO4)3 are generated when 152 g of H2SO4 reacts?
A)530. g of Al2(SO4)3
B)1590 g of Al2(SO4)3
C)177 g of Al2(SO4)3
D)43.6 g of Al2(SO4)3
E)131 g of Al2(SO4)3
A)530. g of Al2(SO4)3
B)1590 g of Al2(SO4)3
C)177 g of Al2(SO4)3
D)43.6 g of Al2(SO4)3
E)131 g of Al2(SO4)3

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
13
The Greek letter delta ( )may be written over the reaction arrow in a chemical equation to indicate that
A)heat is generated when the reaction occurs.
B)a catalyst is needed for the reaction to occur.
C)water is needed for the reaction to occur.
D)heat is needed for the reaction to occur.
A)heat is generated when the reaction occurs.
B)a catalyst is needed for the reaction to occur.
C)water is needed for the reaction to occur.
D)heat is needed for the reaction to occur.

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
14
How many moles of chloroethylene (C2H3Cl)contain 5.47 × 1026 molecules?
A)3.29 × 1050 mol of C2H3Cl
B)5.47 × 1026 mol of C2H3Cl
C)909 mol of C2H3Cl
D)5450 mol of C2H3Cl
A)3.29 × 1050 mol of C2H3Cl
B)5.47 × 1026 mol of C2H3Cl
C)909 mol of C2H3Cl
D)5450 mol of C2H3Cl

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
15
What is the formula weight of Co(NO3)3?
A)88.94 amu
B)244.96 amu
C)216.94 amu
D)148.96 amu
E)196.96 amu
A)88.94 amu
B)244.96 amu
C)216.94 amu
D)148.96 amu
E)196.96 amu

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
16
A balanced chemical equation tells the number of _____ of each reactant that combine and the number of _____ of each product formed.
A)grams, grams
B)grams, moles
C)moles, grams
D)moles, moles
A)grams, grams
B)grams, moles
C)moles, grams
D)moles, moles

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
17
How many carbon atoms are in 3.85 mol of carbon?
A)3.85 carbon atoms
B)23.2 carbon atoms
C)6.02 × 1023 carbon atoms
D)2.32 × 1024 carbon atoms
E)6.40 × 10-24 carbon atoms
A)3.85 carbon atoms
B)23.2 carbon atoms
C)6.02 × 1023 carbon atoms
D)2.32 × 1024 carbon atoms
E)6.40 × 10-24 carbon atoms

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
18
Which chemical equation is properly balanced?
A)SO2 + O2 + H2O H2SO4
B)2 SO2 + O2 + 2 H2O 2 H2SO4
C)SO2 + O2 + 4 H2O 2 H2SO4
D)4 SO2 + O2 + 4 H2O 4 H2SO4
A)SO2 + O2 + H2O H2SO4
B)2 SO2 + O2 + 2 H2O 2 H2SO4
C)SO2 + O2 + 4 H2O 2 H2SO4
D)4 SO2 + O2 + 4 H2O 4 H2SO4

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
19
How many iron atoms are in 0.32 mol of Fe2O3?
A)3.9 × 1023 iron atoms
B)3.9 iron atoms
C)6.02 × 1023 iron atoms
D)1.9 × 1023 iron atoms
E)1.1 × 10-24 iron atoms
A)3.9 × 1023 iron atoms
B)3.9 iron atoms
C)6.02 × 1023 iron atoms
D)1.9 × 1023 iron atoms
E)1.1 × 10-24 iron atoms

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
20
How many moles of sulfur trioxide are formed from 3 moles of sulfur dioxide using the given balanced equation? 2 SO2 + O2 2 SO3
A)1 mol of SO3
B)2 mol of SO3
C)3 mol of SO3
D)5 mol of SO3
E)6 mol of SO3
A)1 mol of SO3
B)2 mol of SO3
C)3 mol of SO3
D)5 mol of SO3
E)6 mol of SO3

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
21
The conversion factor shown below was obtained from the balanced chemical equation: 4 KO2(s)+ 2 CO2(g) 2 K2CO3(s)+ 3 O2(g). This conversion factor would be used to calculate which of the following? 
A)the amount of KO2 required to react with a certain amount of O2
B)the amount of O2 that can react with a certain amount of KO2
C)the amount of O2 that can be produced from a certain amount of KO2
D)the amount of KO2 that is required to produce a certain amount of O2

A)the amount of KO2 required to react with a certain amount of O2
B)the amount of O2 that can react with a certain amount of KO2
C)the amount of O2 that can be produced from a certain amount of KO2
D)the amount of KO2 that is required to produce a certain amount of O2

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
22
How many grams of oxygen gas are required to completely react with 77.28 g of ethane (C2H6)in the balanced redox reaction: 2 C2H6(g)+ 7 O2(g) 4 CO2(g)+ 6 H2O(g)?
A)77.28 g of O2(g)
B)23.50 g of O2(g)
C)143.9 g of O2(g)
D)8.995 g of O2(g)
E)287.8 g of O2(g)
A)77.28 g of O2(g)
B)23.50 g of O2(g)
C)143.9 g of O2(g)
D)8.995 g of O2(g)
E)287.8 g of O2(g)

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
23
Tin (II)fluoride can be made by the following reaction: Sn(s)+ 2 HF(g) SnF2(s)+ H2(g). What is the maximum amount of SnF2 that can be produced when 0.480 moles of Sn are mixed with 0.720 moles of HF?
A)0.360 moles of SnF2
B)0.480 moles of SnF2
C)0.600 moles of SnF2
D)0.720 moles of SnF2
E)1.20 moles of SnF2
A)0.360 moles of SnF2
B)0.480 moles of SnF2
C)0.600 moles of SnF2
D)0.720 moles of SnF2
E)1.20 moles of SnF2

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
24
The chemical equation: KClO3(s) KCl(s)+ O2(g)is not balanced. Balance this equation, then select the statement that best describes the balanced equation.
A)The balanced equation will have six atoms of oxygen on each side of the equation.
B)The balanced equation will have one atom of chlorine on each side of the equation.
C)The balanced equation will have an O3 on the product side of the equation.
D)The balanced equation will have a coefficient of 1 in front of KCl.
A)The balanced equation will have six atoms of oxygen on each side of the equation.
B)The balanced equation will have one atom of chlorine on each side of the equation.
C)The balanced equation will have an O3 on the product side of the equation.
D)The balanced equation will have a coefficient of 1 in front of KCl.

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
25
In the balanced redox reaction: 2 C2H6(g)+ 7 O2(g) 4 CO2(g)+ 6 H2O(g), which species is oxidized?
A)C2H6(g)
B)O2(g)
C)CO2(g)
D)H2O(g)
A)C2H6(g)
B)O2(g)
C)CO2(g)
D)H2O(g)

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
26
What is the molar mass of the compound show below? 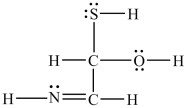
A)90.13 amu
B)90.13 g/mol
C)93.13 amu
D)93.13 g/mol
E)91.14 g/mol

A)90.13 amu
B)90.13 g/mol
C)93.13 amu
D)93.13 g/mol
E)91.14 g/mol

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
27
Identify the species that is oxidized and the species that is reduced in the reaction: 2 I- + Cl2 2 Cl- + I2
A)I- is oxidized and Cl2 is reduced.
B)Cl2 is oxidized and I- is reduced.
C)I2 is oxidized and Cl- is reduced.
D)Cl2 is oxidized and Cl- is reduced.
E)I2 is oxidized and I- is reduced.
A)I- is oxidized and Cl2 is reduced.
B)Cl2 is oxidized and I- is reduced.
C)I2 is oxidized and Cl- is reduced.
D)Cl2 is oxidized and Cl- is reduced.
E)I2 is oxidized and I- is reduced.

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
28
How many moles of sodium chloride (table salt, NaCl, molar mass 58.44 g/mol)are contained in a 1.00 lb box of table salt?
A)26, 500 mol of NaCl
B)4.68 × 1024 mol of NaCl
C)58.44 mol NaCl
D)7.77 mol of NaCl
E)0.129 mol of NaCl
A)26, 500 mol of NaCl
B)4.68 × 1024 mol of NaCl
C)58.44 mol NaCl
D)7.77 mol of NaCl
E)0.129 mol of NaCl

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
29
In the balanced redox reaction: 2 C2H6(g)+ 7 O2(g) 4 CO2(g)+ 6 H2O(g), which species is reduced?
A)C2H6(g)
B)O2(g)
C)CO2(g)
D)H2O(g)
A)C2H6(g)
B)O2(g)
C)CO2(g)
D)H2O(g)

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
30
Potassium metal (K)reacts violently when added to water according to the balanced equation: 2 K(s)+ 2 H2O(l) 2 KOH(aq)+ H2(g). How many moles of H2O are needed to react completely with 7.54 mol of K?
A)2 mol of H2O
B)7.54 mol of H2O
C)15.1 mol of H2O
D)3.77 mol of H2O
A)2 mol of H2O
B)7.54 mol of H2O
C)15.1 mol of H2O
D)3.77 mol of H2O

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
31
Which quantity has the greatest mass?
A)2.0 mol of Na
B)2.0 mol of Na2O
C)2.0 mol of NaCl
D)2.0 mol of O2
E)All of these quantities have the same mass.
A)2.0 mol of Na
B)2.0 mol of Na2O
C)2.0 mol of NaCl
D)2.0 mol of O2
E)All of these quantities have the same mass.

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
32
Consider the oxidation of sodium metal to sodium oxide described by the balanced equation: 4 Na + O2 2 Na2O. If 2.55 mol of sodium reacts, and 75.0 g of Na2O is produced, what is the percent yield?
A)94.9% yield
B)34.0% yield
C)47.5% yield
D)190.% yield
A)94.9% yield
B)34.0% yield
C)47.5% yield
D)190.% yield

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
33
How many carbon atoms are in 77.28 g of ethane (C2H6)?
A)2.570 carbon atoms
B)5.140 carbon atoms
C)3.094 × 1024 carbon atoms
D)1.548 × 1024 carbon atoms
E)1.238 × 1025 carbon atoms
A)2.570 carbon atoms
B)5.140 carbon atoms
C)3.094 × 1024 carbon atoms
D)1.548 × 1024 carbon atoms
E)1.238 × 1025 carbon atoms

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
34
Blimps are essentially very large helium filled balloons. If a blimp contains 536 kg of helium, how many helium atoms are present inside the blimp?
A)134, 000 He atoms
B)8.9 x 10-19 He atoms
C)8.06 x 1028 He atoms
D)3.23 x 1026 He atoms
A)134, 000 He atoms
B)8.9 x 10-19 He atoms
C)8.06 x 1028 He atoms
D)3.23 x 1026 He atoms

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
35
In the balanced redox reaction: 2 Cu(s)+ S(s) Cu2S(s), how many electrons are gained or lost by each copper atom?
A)each copper atom gains two (2)electrons
B)each copper atom gains one (1)electron
C)each copper atom loses one (1)electron
D)each copper atom loses two (2)electrons
A)each copper atom gains two (2)electrons
B)each copper atom gains one (1)electron
C)each copper atom loses one (1)electron
D)each copper atom loses two (2)electrons

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
36
Consider the mixture of Cl2 and F2 in a closed container as illustrated below. What will the contents of the container look like if the molecules undergo the reaction: Cl2(g)+ 3 F2(g) 2 ClF3(g)? 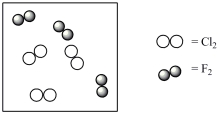
A)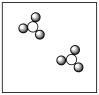
B)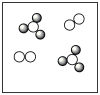
C)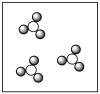
D)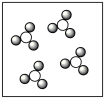

A)

B)

C)

D)


Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
37
If a synthesis has four steps and each step has a 75% yield (0.75 written as a decimal), what is the overall percent yield?
A)75%
B)19%
C)56%
D)32%
E)42%
A)75%
B)19%
C)56%
D)32%
E)42%

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
38
Sodium fluoride can be produced from the reaction between sodium metal and fluorine gas. Which of the following represents the balanced chemical equation for this reaction?
A)2 Na(s)+ F2(g) 2 NaF(s)
B)Na+(s)+ F-(g) NaF(s)
C)Na(s)+ F2(g) NaF2(s)
D)2 Na(s)+ F2(g) Na2F2(s)
A)2 Na(s)+ F2(g) 2 NaF(s)
B)Na+(s)+ F-(g) NaF(s)
C)Na(s)+ F2(g) NaF2(s)
D)2 Na(s)+ F2(g) Na2F2(s)

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
39
What is the mass of 3.4 × 1020 molecules of ethanol (C2H6O)expressed in milligrams?
A)0.026 mg of ethanol
B)26, 000 mg of ethanol
C)0.012 mg of ethanol
D)26 mg of ethanol
A)0.026 mg of ethanol
B)26, 000 mg of ethanol
C)0.012 mg of ethanol
D)26 mg of ethanol

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
40
What are the two half reactions that show how many electrons are gained or lost by each species in the reaction:
Ni2+(aq)+ Mg(s) Ni(s)+ Mg2+(aq)?
A)Ni2+(aq)+ e- Ni(s)and Mg(s) Mg2+(aq)+ e-
B)Ni2+(aq)+ 2 e- Ni(s)and Mg(s) Mg2+(aq)+ 2 e-
C)Ni2+(aq) Ni(s)+ 2 e- and Mg(s)+ 2 e- Mg2+(aq)
D)Ni2+(aq) Ni(s)+ e- and Mg(s)+ e- Mg2+(aq)
E)Ni2+(aq)+ 2 e- Ni(s)and Mg(s)+ 2 e- Mg2+(aq)
Ni2+(aq)+ Mg(s) Ni(s)+ Mg2+(aq)?
A)Ni2+(aq)+ e- Ni(s)and Mg(s) Mg2+(aq)+ e-
B)Ni2+(aq)+ 2 e- Ni(s)and Mg(s) Mg2+(aq)+ 2 e-
C)Ni2+(aq) Ni(s)+ 2 e- and Mg(s)+ 2 e- Mg2+(aq)
D)Ni2+(aq) Ni(s)+ e- and Mg(s)+ e- Mg2+(aq)
E)Ni2+(aq)+ 2 e- Ni(s)and Mg(s)+ 2 e- Mg2+(aq)

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
41
The subscripts in chemical formulas are changed in order to balance a chemical equation.

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
42
A student runs the reaction: LiOH + CO2 LiHCO3. The reaction consumes 45.0 g LiOH and produces 72.8 g of LiHCO3. Which of the statements concerning this reaction is true?
A)The actual yield of the product is 72.8 g LiHCO3.
B)The theoretical yield of the product is 72.8 g LiHCO3.
C)The theoretical yield of the product is 117.8 g LiHCO3.
D)The percent yield of the reaction is 61.8%.
A)The actual yield of the product is 72.8 g LiHCO3.
B)The theoretical yield of the product is 72.8 g LiHCO3.
C)The theoretical yield of the product is 117.8 g LiHCO3.
D)The percent yield of the reaction is 61.8%.

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
43
In the reaction: Ni2+(aq)+ Mg(s) Ni(s)+ Mg2+(aq), the species that undergoes reduction is Mg(s).

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
44
The molar mass of CaCO3 is greater than the molar mass of Ca(NO3)2.

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
45
Combustion reactions are redox reactions.

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
46
In the reaction: Ni2+(aq)+ Mg(s) Ni(s)+ Mg2+(aq), the oxidizing agent is Ni2+(aq).

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
47
All chemical reactions convert one substance into another.

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
48
The balanced reaction: 4 NO2 + O2 + 2 H2O 4 HNO3 states that four grams of nitrogen dioxide reacts with each gram of oxygen.

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
49
Oxidation is the gain of electrons by an atom.

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
50
In calculating the percent yield, both the actual yield and theoretical yield must be in units of grams.

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
51
The actual yield is the amount of product expected from a given amount of reactant based on the coefficients in the balanced chemical equation.

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
52
A chemical equation is balanced by adding coefficients in front of some formulas so that the number of atoms of each element is equal on both sides of the equation.

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
53
A mole of copper atoms has more atoms than a mole of lead atoms.

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
54
One mole of oxygen molecules contains more atoms than one mole of lead atoms.

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
55
Assume that the mixture of substances in Figure 1 undergoes a chemical reaction. Which diagram represents a product mixture that is consistent with the Law of Conservation of Mass? 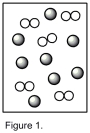
A)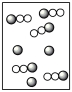
B)
C)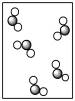
D)It is impossible to predict based on the information given.

A)

B)

C)

D)It is impossible to predict based on the information given.

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
56
The balanced reaction: 4 Fe(s)+ 3 O2(g) 2 Fe2O3(s)is an oxidation-reduction reaction.

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
57
The balanced reaction: 4 NO2 + O2 + 2 H2O 4 HNO3 states that four moles of nitrogen dioxide react with each mole of oxygen.

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
58
Beaker A contains 1 mole of iron atoms, and Beaker B contains 1 mole of lead atoms. Which statement concerning these two samples is known with certainty? 
A)Beakers A and B contain samples with the same molar mass.
B)Beakers A and B contain an equal number of atoms.
C)Beakers A and B contain an equal volume of atoms.
D)Beakers A and B contain equal masses of atoms.

A)Beakers A and B contain samples with the same molar mass.
B)Beakers A and B contain an equal number of atoms.
C)Beakers A and B contain an equal volume of atoms.
D)Beakers A and B contain equal masses of atoms.

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
59
A mole is a quantity that contains 6.02 × 10-23 atoms, molecules, or ions.

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
60
The formula weight of a compound is the sum of the atomic weights of all the atoms in a compound, reported in atomic mass units.

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
61
Consider the unbalanced chemical equation: NH3 + O2 NO + H2O. It requires 55.4 g of NH3 to completely react with 156 g of O2.

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
62
The reaction: Mg(s)+ 2 HBr(aq) MgBr2(s)+ H2(g)is an oxidation-reduction reaction.

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
63
To multiply two numbers in scientific notation, multiply the coefficients together and multiply the exponents in the powers of 10.

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
64
A chemical equation is an expression that uses chemical formulas and other symbols to illustrate what _____ constitute the starting materials in a reaction and what _____ are formed.

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
65
A 100-g sample of the compound below contains less than 6.02 × 1023 molecules. 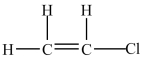
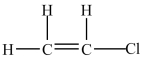

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
66
In the reaction: Ni2+(aq)+ Mg(s) Ni(s)+ Mg2+(aq), two electrons are transferred from Ni2 to Mg.

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
67
Consider the balanced reaction: 2 A + B C, where the molar mass of B is less than the molar mass of A. It requires a smaller mass of A to completely react with given mass of B.

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
68
The actual yield of a product in a chemical reaction should not exceed its theoretical yield.

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
69
The molar mass of dibromomethane (CH2Br2)is larger than the molar mass of dichloromethane (CH2Cl2).

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
70
One term in a balanced chemical equation contains the coefficient 4 in front of the formula Mg3(PO4)2. This term represents that there are 12 Mg atoms, 4 P atoms and 16 O atoms in this term.

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
71
The mass of one ethanol (C2H6O)molecule is 7.65 × 10-23 grams.

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
72
A chemical change alters the chemical composition of a substance, and therefore a new substance is produced.

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
73
The electric current generated in batteries used for portable electronic devices and pacemakers results from redox reactions.

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
74
A 100-g sample of the compound below contains greater than one mole of molecules. 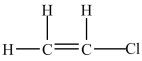
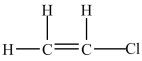

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
75
In a chemical reaction, the actual yield of a product may be reduced by side reactions.

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
76
In redox reactions, metals tend to undergo oxidation.

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
77
Consider the balanced reaction: 4 NO2 + O2 + 2 H2O 4 HNO3. If 100. g of NO2 is placed in a reaction vessel the theoretical yield of nitric acid (HNO3)collected will be 137 g.

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
78
To determine the overall percent yield in a synthesis that has more than one step, subtract the percent yield for each step from 100% yield.

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
79
A 200.-mg ibuprofen (C13H18O2)tablet contains greater than one mole of ibuprofen molecules.

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
80
When the mass of a substance produced in a reaction must be calculated, first its number of moles is determined using mole ratios, and then Avogadro's number is used to convert moles to grams.

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck



