Deck 21: Organic and Biochemical Molecules
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/118
Play
Full screen (f)
Deck 21: Organic and Biochemical Molecules
1
Name the following: 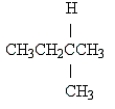
A) dodecane
B) methylpentane
C) isopropane
D) n-pentane
E) methylbutane

A) dodecane
B) methylpentane
C) isopropane
D) n-pentane
E) methylbutane
methylbutane
2
Which of the following, upon reacting with oxygen, would form the greatest amount of carbon dioxide?
A) n-pentane
B) isopentane
C) neopentane
D) Two of these would form equal amounts.
E) All of these would form equal amounts.
A) n-pentane
B) isopentane
C) neopentane
D) Two of these would form equal amounts.
E) All of these would form equal amounts.
All of these would form equal amounts.
3
Which of the following is not a structural isomer of 1-pentene?
A) 2-methyl-2-butene
B) 1-methyl-cyclobutene
C) 2-pentene
D) cyclopentane
E) 3-methyl-1-butene
A) 2-methyl-2-butene
B) 1-methyl-cyclobutene
C) 2-pentene
D) cyclopentane
E) 3-methyl-1-butene
1-methyl-cyclobutene
4
Which of the following has the lowest boiling point?
A) ethane
B) methane
C) propane
D) butane
E) All of these have the same boiling point.
A) ethane
B) methane
C) propane
D) butane
E) All of these have the same boiling point.

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
5
Combustion reactions are substitution reactions with oxygen.

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
6
1-Propene undergoes hydrogenation. The product of this is
A)Methane.
B)Ethane.
C)2-propane.
D)Propane.
E)None of these
A)Methane.
B)Ethane.
C)2-propane.
D)Propane.
E)None of these

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
7
Name the following: 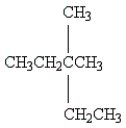
A) 2,2-diethylpropane
B) 2-methyl-2-ethylbutane
C) n-heptane
D) 3,3-dimethylpentane

A) 2,2-diethylpropane
B) 2-methyl-2-ethylbutane
C) n-heptane
D) 3,3-dimethylpentane

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
8
How many isomers are there with the formula C3H4? Include both structural and geometric isomers.
A) 5
B) 2
C) 6
D) 3
E) 4
A) 5
B) 2
C) 6
D) 3
E) 4

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
9
A student gave a molecule the following name: 2-ethyl-3-methyl-5-isopropylhexane
However, his TA pointed out that although the molecule could be correctly drawn from this name, the name violates the systematic rules. What is the correct (IUPAC) name for the molecule?
A) 3,4,6,7-tetramethyloctane
B) 3,4-dimethyl-6-isopropylheptane
C) 1,2-diethyl-3,6,7-trimethylheptane
D) 2,3,5,6-tetramethyloctane
E) 2-isopropyl-4,5-dimethylheptane
However, his TA pointed out that although the molecule could be correctly drawn from this name, the name violates the systematic rules. What is the correct (IUPAC) name for the molecule?
A) 3,4,6,7-tetramethyloctane
B) 3,4-dimethyl-6-isopropylheptane
C) 1,2-diethyl-3,6,7-trimethylheptane
D) 2,3,5,6-tetramethyloctane
E) 2-isopropyl-4,5-dimethylheptane

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
10
Isomers have
A) different molecular formulas and different structures.
B) the same molecular formula and the same structure.
C) the same molecular formula but different structures.
D) different molecular formulas but the same structure.
E) none of these
A) different molecular formulas and different structures.
B) the same molecular formula and the same structure.
C) the same molecular formula but different structures.
D) different molecular formulas but the same structure.
E) none of these

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
11
The product of ethane undergoing dehydrogenation is called
A) ethene.
B) propene.
C) propane.
D) methene.
E) none of these
A) ethene.
B) propene.
C) propane.
D) methene.
E) none of these

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
12
Which of the following names is a correct one?
A) 3,4-dichloropentane
B) 2-fluoro-1-chloro-4,4-dimethylnonane
C) cis-1,3-dimethylbutane
D) 1,1-dimethyl-2,2-diethylbutane
E) 1-fluoro-2,4-methyl-3-propylcyclohexane
A) 3,4-dichloropentane
B) 2-fluoro-1-chloro-4,4-dimethylnonane
C) cis-1,3-dimethylbutane
D) 1,1-dimethyl-2,2-diethylbutane
E) 1-fluoro-2,4-methyl-3-propylcyclohexane

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
13
What is the compound whose carbon skeleton (minus any hydrogen atoms) appears below? 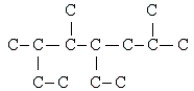
A) 2,4-diethyl-3,6-dimethylheptane
B) 5-ethyl-3,4,6-trimethyloctane
C) 1,4-diethyl-3,6-dimethyl-tridecane
D) 2,5-dimethyl-4,6-diethylheptane
E) 4-ethyl-2,5,6-trimethyloctane
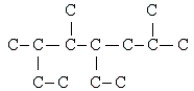
A) 2,4-diethyl-3,6-dimethylheptane
B) 5-ethyl-3,4,6-trimethyloctane
C) 1,4-diethyl-3,6-dimethyl-tridecane
D) 2,5-dimethyl-4,6-diethylheptane
E) 4-ethyl-2,5,6-trimethyloctane

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
14
How many structural isomers does pentane have?
A) 3
B) 5
C) 1
D) 2
E) 4
A) 3
B) 5
C) 1
D) 2
E) 4

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
15
In lecture, the professor named a molecule 2-ethyl-4-tertiary-butylpentane. An alert student pointed out that although the correct structure could be drawn from this name, the name did not follow systematic rules. What is the correct systematic name for the molecule?
A) 3,5,6,6-tetramethylheptane
B) 2-ethyl-4,5,5-trimethylhexane
C) undecane
D) 2,2,3,5-tetramethylheptane
E) 2-t-butyl-5-methylhexane
A) 3,5,6,6-tetramethylheptane
B) 2-ethyl-4,5,5-trimethylhexane
C) undecane
D) 2,2,3,5-tetramethylheptane
E) 2-t-butyl-5-methylhexane

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
16
How many isomers of C4H10 are there?
A) 5
B) 6
C) 2
D) 3
E) 4
A) 5
B) 6
C) 2
D) 3
E) 4

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
17
The compound below is the carbon skeleton (minus any hydrogen atoms) of 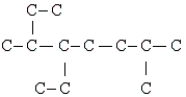
I. a C12H26
II.A substituted octane
III.A compound with 3 tertiary carbons
IV.A compound with 3 secondary carbons
V.A compound with 2 isopropyl groups
A) II, IV, V
B) II, III, IV
C) I, II, III, IV
D) III, IV, V
E) I, II, III
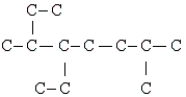
I. a C12H26
II.A substituted octane
III.A compound with 3 tertiary carbons
IV.A compound with 3 secondary carbons
V.A compound with 2 isopropyl groups
A) II, IV, V
B) II, III, IV
C) I, II, III, IV
D) III, IV, V
E) I, II, III

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
18
Name the following: 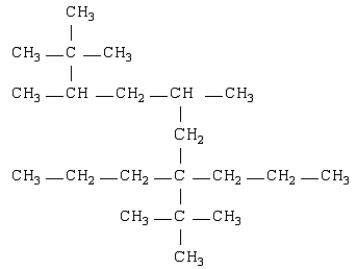
A) 2,2,5,7,8,8-hexamethyl-3,3-dipropylnonane
B) isonanane
C) 6-propyl-2,6-di-t-butylnonane
D) 2,2,3,5-tetramethyl-7-propyl-7-t-butyldecane
E) none of these

A) 2,2,5,7,8,8-hexamethyl-3,3-dipropylnonane
B) isonanane
C) 6-propyl-2,6-di-t-butylnonane
D) 2,2,3,5-tetramethyl-7-propyl-7-t-butyldecane
E) none of these

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
19
Name the following: 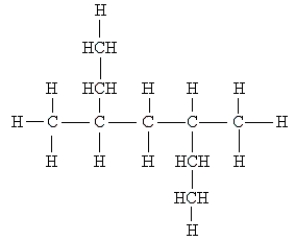
A) 3,5-dimethylheptane
B) secondary ethylpentane
C) 2,4-diethylpentane
D) 2,3-dimethyl-2,3-diethylpropane
E) none of these

A) 3,5-dimethylheptane
B) secondary ethylpentane
C) 2,4-diethylpentane
D) 2,3-dimethyl-2,3-diethylpropane
E) none of these

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
20
A student gave a molecule the following name: 3-methyl-4-isopropylpentane
However, the teacher pointed out that although the molecule could be correctly drawn from this name, the name violates the IUPAC rules. What is the correct (IUPAC) name for the molecule?
A) 4-isopropyl-3-methylpentane
B) 2-isopropyl-3-methylpentane
C) 3,4-dimethylheptane
D) 2,3,4-trimethylhexane
E) 1,1,2,3-tetramethylpentane
However, the teacher pointed out that although the molecule could be correctly drawn from this name, the name violates the IUPAC rules. What is the correct (IUPAC) name for the molecule?
A) 4-isopropyl-3-methylpentane
B) 2-isopropyl-3-methylpentane
C) 3,4-dimethylheptane
D) 2,3,4-trimethylhexane
E) 1,1,2,3-tetramethylpentane

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
21
Which of the following is known as rubbing alcohol?
A) isopropanol
B) methanol
C) ethanol
D) propanol
E) none of these
A) isopropanol
B) methanol
C) ethanol
D) propanol
E) none of these

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
22
In which of the following lists do all members have a C=O bond?
A) secondary alcohol, ketone, aldehyde
B) ester, aldehyde, secondary alcohol, ketone
C) ester, aldehyde, ketone
D) any alcohol, ether, ester
E) carboxylic acid, ether, tertiary alcohol
A) secondary alcohol, ketone, aldehyde
B) ester, aldehyde, secondary alcohol, ketone
C) ester, aldehyde, ketone
D) any alcohol, ether, ester
E) carboxylic acid, ether, tertiary alcohol

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
23
Which of the following often have useful solvent properties?
A) esters only
B) ketones only
C) alcohols only
D) amines only
E) All of these have useful solvent properties.
A) esters only
B) ketones only
C) alcohols only
D) amines only
E) All of these have useful solvent properties.

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
24
The oxidation of secondary alcohols results in
A) secondary alcohols.
B) aldehydes.
C) ketones.
D) esters.
E) ethers.
A) secondary alcohols.
B) aldehydes.
C) ketones.
D) esters.
E) ethers.

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
25
Which of the following have a -C-O-C- functional group?
A) esters
B) alcohols
C) aldehydes
D) ethers
E) amines
A) esters
B) alcohols
C) aldehydes
D) ethers
E) amines

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
26
How many different possible tetramethylbenzenes exist?
A) 6
B) 2
C) 5
D) 3
E) 4
A) 6
B) 2
C) 5
D) 3
E) 4

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
27
Name the following: 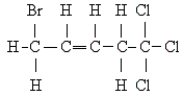
A) 1,1,1-trichloro-5-bromo-3-pentyne
B) 1,1,1-trichloro-5-bromo-3-pentene
C) 5,5,5-trichloro-1-bromo-2-pentene
D) 1,1,1-trichloro-5-bromo-2-pentene
E) none of these
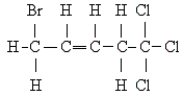
A) 1,1,1-trichloro-5-bromo-3-pentyne
B) 1,1,1-trichloro-5-bromo-3-pentene
C) 5,5,5-trichloro-1-bromo-2-pentene
D) 1,1,1-trichloro-5-bromo-2-pentene
E) none of these

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
28
For which of the following compound(s) are cis and trans isomers possible?
A) dibromoethyne
B) 2,3-dichloro-2-butene
C) 3,4-diethyl-3-hexene
D) ortho-chlorotoluene
E) 4,4-dimethylcyclohexanol
A) dibromoethyne
B) 2,3-dichloro-2-butene
C) 3,4-diethyl-3-hexene
D) ortho-chlorotoluene
E) 4,4-dimethylcyclohexanol

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
29
Name the following: 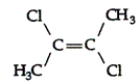
A) 2,3-dichloro-1-methyl-propene
B) 2,3-dichloro-trans-2-butene
C) 2,3-dichloro-cis-2-butene
D) 2-chloro-3-chloro-cis-2-butene
E) 1-chloro-1-methyl-2-chloro-propene

A) 2,3-dichloro-1-methyl-propene
B) 2,3-dichloro-trans-2-butene
C) 2,3-dichloro-cis-2-butene
D) 2-chloro-3-chloro-cis-2-butene
E) 1-chloro-1-methyl-2-chloro-propene

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
30
Consider the molecule trans-2-butene. Which statement is true?
A) There is free rotation around every bond in the molecule.
B) Cis-2-butene is its structural isomer.
C) Carbon #2 exhibits sp2 hybridization.
D) The molecule has two bonds.
E) none of these
A) There is free rotation around every bond in the molecule.
B) Cis-2-butene is its structural isomer.
C) Carbon #2 exhibits sp2 hybridization.
D) The molecule has two bonds.
E) none of these

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
31
Which of the following is known as wood alcohol?
A) methanol
B) isopropanol
C) ethanol
D) propanol
E) none of these
A) methanol
B) isopropanol
C) ethanol
D) propanol
E) none of these

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
32
Consider the following four compounds: 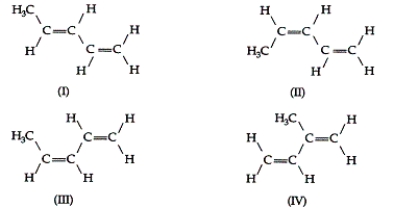 Which of these compounds would have the same physical properties (melting point, boiling point, density, and so on)?
Which of these compounds would have the same physical properties (melting point, boiling point, density, and so on)?
A) I and IV
B) I and II
C) I and III
D) III and IV
E) II and III
 Which of these compounds would have the same physical properties (melting point, boiling point, density, and so on)?
Which of these compounds would have the same physical properties (melting point, boiling point, density, and so on)?A) I and IV
B) I and II
C) I and III
D) III and IV
E) II and III

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
33
Name the following: 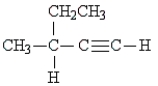
A) 1-hexyne
B) 2-ethyl-3-butyne
C) 3-methyl-1-pentyne
D) 2-ethynyl butane
E) 3-methyl-4-pentyne

A) 1-hexyne
B) 2-ethyl-3-butyne
C) 3-methyl-1-pentyne
D) 2-ethynyl butane
E) 3-methyl-4-pentyne

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
34
Which of the following compounds can exhibit geometric isomerism?
A)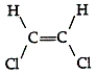
B)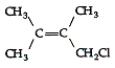
C)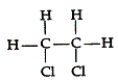
D)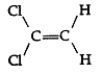
E)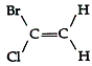
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
35
How many of the following molecules exist?
I.Methene
II.Cycloethane
III.Isopropyne
IV.Neobutane
A) 2
B) 1
C) 4
D) 3
E) 0
I.Methene
II.Cycloethane
III.Isopropyne
IV.Neobutane
A) 2
B) 1
C) 4
D) 3
E) 0

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
36
H2CCHCH2N(CH3)2 is
A) an alkyne and a tertiary amine.
B) an alkyne and a secondary amine.
C) an alkene and a primary amine.
D) an alkene and a tertiary amine.
E) none of these
A) an alkyne and a tertiary amine.
B) an alkyne and a secondary amine.
C) an alkene and a primary amine.
D) an alkene and a tertiary amine.
E) none of these

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
37
The common name for 2-propanol is
A) n-propyl alcohol.
B) methanol.
C) ethanol.
D) isopropyl alcohol.
E) none of these
A) n-propyl alcohol.
B) methanol.
C) ethanol.
D) isopropyl alcohol.
E) none of these

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
38
Which of the following types of compounds lacks an sp2-hybridized carbon center?
A) ketones
B) aldehydes
C) alkenes
D) alcohols
E) benzene
A) ketones
B) aldehydes
C) alkenes
D) alcohols
E) benzene

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
39
Which of the following is an incorrect name?
A) ethylene
B) propyne
C) trans-1,2-dichloropropene
D) 1,1-dichloropropane
E) cis-1,2-dichloropropane
A) ethylene
B) propyne
C) trans-1,2-dichloropropene
D) 1,1-dichloropropane
E) cis-1,2-dichloropropane

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
40
How many structural and geometric isomers are there of chloropropene?
A) 2
B) 3
C) more than 5
D) 5
E) 4
A) 2
B) 3
C) more than 5
D) 5
E) 4

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
41
Classify the following molecule: 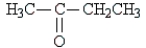
A) aldehyde
B) carbonyl
C) acid
D) ketone
E) amine
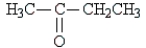
A) aldehyde
B) carbonyl
C) acid
D) ketone
E) amine

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
42
Identify the type of organic compound shown: 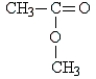
A) aldehyde
B) amine
C) ketone
D) ester
E) none of these

A) aldehyde
B) amine
C) ketone
D) ester
E) none of these

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
43
Name the following: 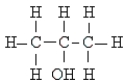
A) methyl alcohol
B) butanol
C) ethyl alcohol
D) propyl alcohol
E) isopropyl alcohol

A) methyl alcohol
B) butanol
C) ethyl alcohol
D) propyl alcohol
E) isopropyl alcohol

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
44
Name the following: 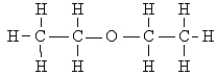
A) acetone
B) diethyl ether
C) diethylketone
D) butyraldehyde
E) none of these

A) acetone
B) diethyl ether
C) diethylketone
D) butyraldehyde
E) none of these

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
45
Refer to the following structures. Which of the statements below is true of them? 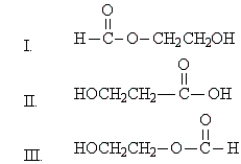
A) I and II have different molecular formulas.
B) II and III are stereoisomers of each other.
C) I and III are the same compound.
D) I and III are structural isomers of each other.
E) II and III are different conformations of the same compound.

A) I and II have different molecular formulas.
B) II and III are stereoisomers of each other.
C) I and III are the same compound.
D) I and III are structural isomers of each other.
E) II and III are different conformations of the same compound.

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
46
Which structure represents an optically active aldehyde?
A)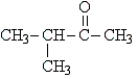
B)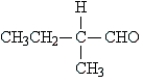
C)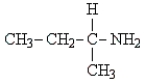
D)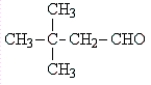
E)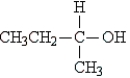
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
47
Which of the following will yield a carboxylic acid upon oxidation?
A) a ketone
B) a cycloalkane
C) an aldehyde
D) tertiary alcohol
E) a secondary alcohol
A) a ketone
B) a cycloalkane
C) an aldehyde
D) tertiary alcohol
E) a secondary alcohol

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
48
Identify the type of organic compound shown: (CH3)2CHOH
A) carboxylic acid
B) ether
C) secondary alcohol
D) tertiary alcohol
E) primary amine
A) carboxylic acid
B) ether
C) secondary alcohol
D) tertiary alcohol
E) primary amine

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
49
Identify the type of organic compound shown: 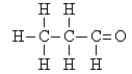
A) amine
B) ester
C) ketone
D) aldehyde
E) none of these
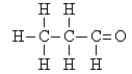
A) amine
B) ester
C) ketone
D) aldehyde
E) none of these

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
50
Which of the following becomes more soluble in water upon the addition of NaOH?
A) an alkane
B) an aromatic hydrocarbon
C) an amine
D) a carboxylic acid
E) an amide
A) an alkane
B) an aromatic hydrocarbon
C) an amine
D) a carboxylic acid
E) an amide

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
51
If you were to heat pentanoic acid and 2-butanol with an acid catalyst, which of the following would you be most likely discover in your flask?
A) an alkane
B) a ketone
C) an ester
D) an amine
E) an aldehyde
A) an alkane
B) a ketone
C) an ester
D) an amine
E) an aldehyde

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
52
Name the following: 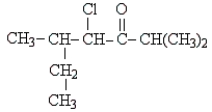
A) 3-methyl-4-chloro-1-isopropylpentanone
B) 4-chloro-2,5-dimethyl-3-heptanone
C) 2-chloro-3-ethyl-1-isopropylbutanone
D) 2-butyl,chloro,isobutanoyl methane
E) isopropyl-chloro,methylbutyl ketone

A) 3-methyl-4-chloro-1-isopropylpentanone
B) 4-chloro-2,5-dimethyl-3-heptanone
C) 2-chloro-3-ethyl-1-isopropylbutanone
D) 2-butyl,chloro,isobutanoyl methane
E) isopropyl-chloro,methylbutyl ketone

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
53
Classify the following molecule: 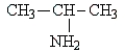
A) tertiary amine
B) secondary amine
C) primary amine
D) amino acid
E) peptide
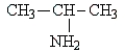
A) tertiary amine
B) secondary amine
C) primary amine
D) amino acid
E) peptide

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
54
Classify the following molecule: 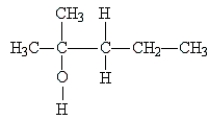
A) primary alcohol
B) tertiary alcohol
C) ether
D) secondary alcohol
E) phenol

A) primary alcohol
B) tertiary alcohol
C) ether
D) secondary alcohol
E) phenol

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
55
Name the following: 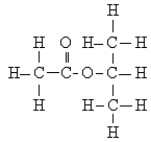
A) ethyl propanoate
B) n-propyl acetate
C) isopropyl formate
D) isopropyl acetate
E) none of these

A) ethyl propanoate
B) n-propyl acetate
C) isopropyl formate
D) isopropyl acetate
E) none of these

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
56
Classify the following molecule: 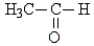
A) amine
B) acid
C) ketone
D) aldehyde
E) carbonyl
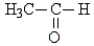
A) amine
B) acid
C) ketone
D) aldehyde
E) carbonyl

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
57
Which of the following is found in beverages such as wine?
A) propanol
B) isopropanol
C) ethanol
D) methanol
E) none of these
A) propanol
B) isopropanol
C) ethanol
D) methanol
E) none of these

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
58
When the following organic compound is oxidized, what is the major organic product? (CH3CH2)2CHOH + KMnO4
A) 3-pentanone
B) 3-pentanal
C) 3-pentanoic acid
D) diethylether
E) 3-pentanol
A) 3-pentanone
B) 3-pentanal
C) 3-pentanoic acid
D) diethylether
E) 3-pentanol

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
59
Oxidation of 2-methyl-1-butanol could yield 
A) II only
B) II and III
C) I and III
D) I only
E) III only

A) II only
B) II and III
C) I and III
D) I only
E) III only

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
60
Identify the secondary amine.
A)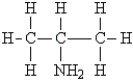
B) CH3CH2NH2
C) (CH3)2NCH2CH3
D) (CH3)2NH
E) NH3
A)

B) CH3CH2NH2
C) (CH3)2NCH2CH3
D) (CH3)2NH
E) NH3

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
61
Pick the optically active molecule among the following:
A)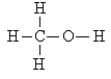
B)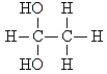
C)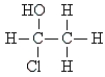
D)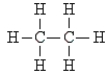
E) none of these
A)

B)

C)

D)

E) none of these

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
62
Teflon is an example of a
A) copolymer.
B) homopolymer.
C) dimer.
D) two of these
E) none of these
A) copolymer.
B) homopolymer.
C) dimer.
D) two of these
E) none of these

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
63
Hydrogen bonding between -C=O groups and NH- groups in the backbone of a protein determines the
A) tertiary structure.
B) secondary structure.
C) quaternary structure.
D) primary structure.
E) all of these
A) tertiary structure.
B) secondary structure.
C) quaternary structure.
D) primary structure.
E) all of these

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
64
The analysis of a protein for its amino acid content is valuable in determining the protein's
A) quaternary structure.
B) secondary structure.
C) primary structure.
D) tertiary structure.
A) quaternary structure.
B) secondary structure.
C) primary structure.
D) tertiary structure.

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
65
Which of the following is the best description of a protein?
A) a chain of amino acids formed by condensation polymerization
B) an alternating chain of amino acids and nucleic acids
C) two antiparallel chains of nucleic acids connected by hydrogen bonding
D) a chain of amino acids connected by ester bonds
E) a chain of nucleotides connected by phosphodiester bonds
A) a chain of amino acids formed by condensation polymerization
B) an alternating chain of amino acids and nucleic acids
C) two antiparallel chains of nucleic acids connected by hydrogen bonding
D) a chain of amino acids connected by ester bonds
E) a chain of nucleotides connected by phosphodiester bonds

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
66
A polypeptide is
A) an addition polymer of amino acids.
B) a part of nucleic acids.
C) a polymer of sugar molecules.
D) a condensation polymer of amino acids.
E) none of these
A) an addition polymer of amino acids.
B) a part of nucleic acids.
C) a polymer of sugar molecules.
D) a condensation polymer of amino acids.
E) none of these

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
67
Oxidation of a primary alcohol results in a(n) __________, and oxidation of a secondary alcohol results in a(n) _________.
A) ester, ether
B) ketone, aldehyde
C) amine, carboxylic acid
D) carboxylic acid, amine
E) aldehyde, ketone
A) ester, ether
B) ketone, aldehyde
C) amine, carboxylic acid
D) carboxylic acid, amine
E) aldehyde, ketone

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
68
Which of the following is optically active (that is, chiral)?
A) 2-chloropropane
B) 1-bromohexane
C) 2-chlorobutane
D) difluoromethane
E) dimethylamine
A) 2-chloropropane
B) 1-bromohexane
C) 2-chlorobutane
D) difluoromethane
E) dimethylamine

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
69
When C4H8 is treated with water and H2SO4, a tertiary alcohol is produced. Which of the following structures could represent C4H8 in this reaction?
A) CH3CH2CH = CH2
B) CH3CH = CHCH3
C)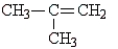
D) CH3CH2CH2CH3
E) none of these
A) CH3CH2CH = CH2
B) CH3CH = CHCH3
C)
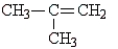
D) CH3CH2CH2CH3
E) none of these

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
70
The condensation product of two amino acids is a(n)
A) ester.
B) peptide.
C) alcohol.
D) ketone.
E) ether.
A) ester.
B) peptide.
C) alcohol.
D) ketone.
E) ether.

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
71
Which of the following molecules exhibits chirality?
A) CH3NH2
B) CH2Cl2
C) CH3CH2OCH3
D)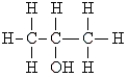
E) CH3CClFOH
A) CH3NH2
B) CH2Cl2
C) CH3CH2OCH3
D)
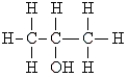
E) CH3CClFOH

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
72
No atoms are lost from starting material in making which kind of polymer?
A) vulcanized polymer
B) addition polymer
C) condensation polymer
D) branched polymer
E) polyester polymer
A) vulcanized polymer
B) addition polymer
C) condensation polymer
D) branched polymer
E) polyester polymer

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
73
The overall shape of a protein is maintained by
A) hydrogen bonding.
B) ionic bonds.
C) dipole-dipole bonding.
D) covalent bonds.
E) all of these
A) hydrogen bonding.
B) ionic bonds.
C) dipole-dipole bonding.
D) covalent bonds.
E) all of these

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
74
The structures of proteins are partially determined by the order of various amino acids in the macromolecule. This level of structural determination is known as
A) tertiary structure.
B) secondary structure.
C) the order of bases.
D) quaternary structure.
E) primary structure.
A) tertiary structure.
B) secondary structure.
C) the order of bases.
D) quaternary structure.
E) primary structure.

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
75
The boiling point of methanol is much higher than that of ethane. This is primarily due to
A) the significant molecular size difference between methanol and ethane.
B) the difference between the molar mass of methanol and that of ethane.
C) the carbon-oxygen double bond in the methanol.
D) the hydrogen bonding in methanol.
E) none of these
A) the significant molecular size difference between methanol and ethane.
B) the difference between the molar mass of methanol and that of ethane.
C) the carbon-oxygen double bond in the methanol.
D) the hydrogen bonding in methanol.
E) none of these

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
76
A protein is
A) a polysaccharide.
B) a polymer of amino acid units.
C) a saturated ester of glycerol.
D) one of the units composing a nucleic acid.
E) an aromatic hydrocarbon with a fused ring structure.
A) a polysaccharide.
B) a polymer of amino acid units.
C) a saturated ester of glycerol.
D) one of the units composing a nucleic acid.
E) an aromatic hydrocarbon with a fused ring structure.

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
77
The alpha helix of a protein is held in a coiled conformation partly because of
A) hydrogen bonding.
B) double bonding.
C) active sites.
D) optical activity.
A) hydrogen bonding.
B) double bonding.
C) active sites.
D) optical activity.

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
78
Which of the following yields a primary alcohol upon reduction?
A) a ketone
B) an amine
C) an alkene
D) an aldehyde
E) an ether
A) a ketone
B) an amine
C) an alkene
D) an aldehyde
E) an ether

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
79
Aspirin is formed via a(n) __________ reaction.
A) combustion
B) hydrogenation
C) condensation
D) substitution
E) addition
A) combustion
B) hydrogenation
C) condensation
D) substitution
E) addition

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
80
An example of a secondary structure of a protein is
A) a peptide linkage.
B) a pleated sheet.
C) serine.
D) an alpha amino acid.
E) none of these
A) a peptide linkage.
B) a pleated sheet.
C) serine.
D) an alpha amino acid.
E) none of these

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck



