Deck 6: Chemical Equilibrium
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/78
Play
Full screen (f)
Deck 6: Chemical Equilibrium
1
The value of the equilibrium constant K depends on:
i.the initial concentrations of the reactants.II.the initial concentrations of the products.III.the final concentrations of the reactants.IV.the final concentrations of the products.
A) II and III only
B) III and IV only
C) I and II only
D) three of these
E) none of these
i.the initial concentrations of the reactants.II.the initial concentrations of the products.III.the final concentrations of the reactants.IV.the final concentrations of the products.
A) II and III only
B) III and IV only
C) I and II only
D) three of these
E) none of these
none of these
2
Which of the following statements is true?
A) When two opposing processes are proceeding at identical rates, the system is at equilibrium.
B) An endothermic reaction shifts toward reactants when heat is added to the reaction.
C) The concentration of the products equals that of the reactants and is constant at equilibrium.
D) Catalysts are an effective means of changing the position of an equilibrium.
E) None of the above statements is true.
A) When two opposing processes are proceeding at identical rates, the system is at equilibrium.
B) An endothermic reaction shifts toward reactants when heat is added to the reaction.
C) The concentration of the products equals that of the reactants and is constant at equilibrium.
D) Catalysts are an effective means of changing the position of an equilibrium.
E) None of the above statements is true.
When two opposing processes are proceeding at identical rates, the system is at equilibrium.
3
For the reaction below, Kp = 1.16 at 890.°C.
CaCO3(s) ⇌ CaO(s) + CO2(g)
If a 22.0-g sample of CaCO3 is put into a 10.4-L container and heated to 890.°C, what percent of the CaCO3 will react to reach equilibrium?
A)12.6%
B)25.3%
C)57.5%
D)100.%
E) 54.9%
CaCO3(s) ⇌ CaO(s) + CO2(g)
If a 22.0-g sample of CaCO3 is put into a 10.4-L container and heated to 890.°C, what percent of the CaCO3 will react to reach equilibrium?
A)12.6%
B)25.3%
C)57.5%
D)100.%
E) 54.9%
57.5%
4
Consider the reaction
CaCl2(s) + 2H2O(g)![<strong>Consider the reaction CaCl<sub>2</sub>(s) + 2H<sub>2</sub>O(g) CaCl<sub>2</sub>•2H<sub>2</sub>O(s) What is the equilibrium constant for the reaction as written?</strong> A) K = [H<sub>2</sub>O]<sup>2</sup> B) K = C) K = D) K = E) K =](https://d2lvgg3v3hfg70.cloudfront.net/TB6422/11eaaf91_9e31_96e4_892c_8529d896ac23_TB6422_11.jpg) CaCl2•2H2O(s)
CaCl2•2H2O(s)
What is the equilibrium constant for the reaction as written?
A) K = [H2O]2
B) K =![<strong>Consider the reaction CaCl<sub>2</sub>(s) + 2H<sub>2</sub>O(g) CaCl<sub>2</sub>•2H<sub>2</sub>O(s) What is the equilibrium constant for the reaction as written?</strong> A) K = [H<sub>2</sub>O]<sup>2</sup> B) K = C) K = D) K = E) K =](https://d2lvgg3v3hfg70.cloudfront.net/TB6422/11eaaf91_9e31_96e5_892c_094029ee6fc8_TB6422_11.jpg)
C) K =![<strong>Consider the reaction CaCl<sub>2</sub>(s) + 2H<sub>2</sub>O(g) CaCl<sub>2</sub>•2H<sub>2</sub>O(s) What is the equilibrium constant for the reaction as written?</strong> A) K = [H<sub>2</sub>O]<sup>2</sup> B) K = C) K = D) K = E) K =](https://d2lvgg3v3hfg70.cloudfront.net/TB6422/11eaaf91_9e31_bdf6_892c_7dc8fa30f6b0_TB6422_11.jpg)
D) K =![<strong>Consider the reaction CaCl<sub>2</sub>(s) + 2H<sub>2</sub>O(g) CaCl<sub>2</sub>•2H<sub>2</sub>O(s) What is the equilibrium constant for the reaction as written?</strong> A) K = [H<sub>2</sub>O]<sup>2</sup> B) K = C) K = D) K = E) K =](https://d2lvgg3v3hfg70.cloudfront.net/TB6422/11eaaf91_9e31_bdf7_892c_1d2984c269da_TB6422_11.jpg)
E) K =![<strong>Consider the reaction CaCl<sub>2</sub>(s) + 2H<sub>2</sub>O(g) CaCl<sub>2</sub>•2H<sub>2</sub>O(s) What is the equilibrium constant for the reaction as written?</strong> A) K = [H<sub>2</sub>O]<sup>2</sup> B) K = C) K = D) K = E) K =](https://d2lvgg3v3hfg70.cloudfront.net/TB6422/11eaaf91_9e31_bdf8_892c_c5e5d74d2f82_TB6422_11.jpg)
CaCl2(s) + 2H2O(g)
![<strong>Consider the reaction CaCl<sub>2</sub>(s) + 2H<sub>2</sub>O(g) CaCl<sub>2</sub>•2H<sub>2</sub>O(s) What is the equilibrium constant for the reaction as written?</strong> A) K = [H<sub>2</sub>O]<sup>2</sup> B) K = C) K = D) K = E) K =](https://d2lvgg3v3hfg70.cloudfront.net/TB6422/11eaaf91_9e31_96e4_892c_8529d896ac23_TB6422_11.jpg) CaCl2•2H2O(s)
CaCl2•2H2O(s)What is the equilibrium constant for the reaction as written?
A) K = [H2O]2
B) K =
![<strong>Consider the reaction CaCl<sub>2</sub>(s) + 2H<sub>2</sub>O(g) CaCl<sub>2</sub>•2H<sub>2</sub>O(s) What is the equilibrium constant for the reaction as written?</strong> A) K = [H<sub>2</sub>O]<sup>2</sup> B) K = C) K = D) K = E) K =](https://d2lvgg3v3hfg70.cloudfront.net/TB6422/11eaaf91_9e31_96e5_892c_094029ee6fc8_TB6422_11.jpg)
C) K =
![<strong>Consider the reaction CaCl<sub>2</sub>(s) + 2H<sub>2</sub>O(g) CaCl<sub>2</sub>•2H<sub>2</sub>O(s) What is the equilibrium constant for the reaction as written?</strong> A) K = [H<sub>2</sub>O]<sup>2</sup> B) K = C) K = D) K = E) K =](https://d2lvgg3v3hfg70.cloudfront.net/TB6422/11eaaf91_9e31_bdf6_892c_7dc8fa30f6b0_TB6422_11.jpg)
D) K =
![<strong>Consider the reaction CaCl<sub>2</sub>(s) + 2H<sub>2</sub>O(g) CaCl<sub>2</sub>•2H<sub>2</sub>O(s) What is the equilibrium constant for the reaction as written?</strong> A) K = [H<sub>2</sub>O]<sup>2</sup> B) K = C) K = D) K = E) K =](https://d2lvgg3v3hfg70.cloudfront.net/TB6422/11eaaf91_9e31_bdf7_892c_1d2984c269da_TB6422_11.jpg)
E) K =
![<strong>Consider the reaction CaCl<sub>2</sub>(s) + 2H<sub>2</sub>O(g) CaCl<sub>2</sub>•2H<sub>2</sub>O(s) What is the equilibrium constant for the reaction as written?</strong> A) K = [H<sub>2</sub>O]<sup>2</sup> B) K = C) K = D) K = E) K =](https://d2lvgg3v3hfg70.cloudfront.net/TB6422/11eaaf91_9e31_bdf8_892c_c5e5d74d2f82_TB6422_11.jpg)

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
5
For the reaction below, Kp = 1.16 at 890.°C.CaCO3(s) 
CaO(s) + CO2(g)
If a 22.0-g sample of CaCO3 is put into a 10.4-L container and heated to 890.°C, what percent of the CaCO3 will react to reach equilibrium?
a.
12.6%
b.
25.3%
c.
57.5%
d.

CaO(s) + CO2(g)
If a 22.0-g sample of CaCO3 is put into a 10.4-L container and heated to 890.°C, what percent of the CaCO3 will react to reach equilibrium?
a.
12.6%
b.
25.3%
c.
57.5%
d.

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
6
Consider the equation 2NOCl2(g)  2NO(g) + Cl2(g). The equilibrium constant is0.0142 at 117°C. Calculate Kp.
2NO(g) + Cl2(g). The equilibrium constant is0.0142 at 117°C. Calculate Kp.
A) 0.0142
B) 0.136
C) 0.454
D) 4.44 × 10-4
E) 46.0
 2NO(g) + Cl2(g). The equilibrium constant is0.0142 at 117°C. Calculate Kp.
2NO(g) + Cl2(g). The equilibrium constant is0.0142 at 117°C. Calculate Kp.A) 0.0142
B) 0.136
C) 0.454
D) 4.44 × 10-4
E) 46.0

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
7
Choose the mass action or equilibrium expression for the reaction
2SO2(g) + O2(g) 2SO3(g)
2SO3(g)
A)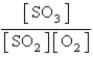
B)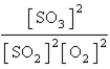
C)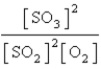
D)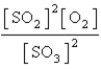
E) none of these
2SO2(g) + O2(g)
 2SO3(g)
2SO3(g)A)
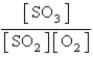
B)
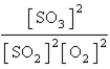
C)
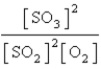
D)
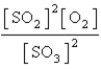
E) none of these

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
8
If, at a given temperature, the equilibrium constant for the reaction H2(g) + Cl2(g) 
2HCl(g) is 5.0, then the equilibrium constant for the reaction HCl(g)
(1/2)H2(g) + (1/2)Cl2(g) can be represented as
a.
0.040.
b.

2HCl(g) is 5.0, then the equilibrium constant for the reaction HCl(g)

(1/2)H2(g) + (1/2)Cl2(g) can be represented as
a.
0.040.
b.

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
9
For the reaction 2NCl3(g)  N2(g) + 3Cl2(g), the equilibrium pressures are
N2(g) + 3Cl2(g), the equilibrium pressures are
P(NCl3) = 0.136 atm
P(N2) = 2.32 atm
P(Cl2) = 0.0580 atm
Determine Kp for this reaction.
A) 0.0245
B) 40.9
C) 9.17
D) 1.48
E) 0.989
 N2(g) + 3Cl2(g), the equilibrium pressures are
N2(g) + 3Cl2(g), the equilibrium pressures areP(NCl3) = 0.136 atm
P(N2) = 2.32 atm
P(Cl2) = 0.0580 atm
Determine Kp for this reaction.
A) 0.0245
B) 40.9
C) 9.17
D) 1.48
E) 0.989

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
10
Which of the following statements concerning equilibrium is not true?
A) A system that is disturbed from an equilibrium condition responds in such a way as to restore equilibrium.
B) The value of the equilibrium constant for a given reaction mixture is the same regardless of the direction from which equilibrium is attained.
C) Equilibrium in molecular systems is dynamic, with two opposing processes balancing one another.
D) The equilibrium constant is independent of temperature.
E) A system moves spontaneously toward a state of equilibrium.
A) A system that is disturbed from an equilibrium condition responds in such a way as to restore equilibrium.
B) The value of the equilibrium constant for a given reaction mixture is the same regardless of the direction from which equilibrium is attained.
C) Equilibrium in molecular systems is dynamic, with two opposing processes balancing one another.
D) The equilibrium constant is independent of temperature.
E) A system moves spontaneously toward a state of equilibrium.

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
11
The value of the equilibrium constant K is dependent on:
I.the temperature of the system.
II.the nature of the reactants and products.
III.the concentration of the reactants.
IV.the concentration of the products.
A) I and II only
B) III and IV only
C) II and III only
D) three of these
E) none of these
I.the temperature of the system.
II.the nature of the reactants and products.
III.the concentration of the reactants.
IV.the concentration of the products.
A) I and II only
B) III and IV only
C) II and III only
D) three of these
E) none of these

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
12
The value of Kp for the reaction H2(g) + O2(g)  H2O2(g) is 2.3 × 106 at 610K. Determine the value for K for this reaction at 610K.
H2O2(g) is 2.3 × 106 at 610K. Determine the value for K for this reaction at 610K.
A) 1.2 × 1010
B) 4.6 × 104
C) 1.2 × 108
D) 2.3 × 106
E) 4.3 × 10-7
 H2O2(g) is 2.3 × 106 at 610K. Determine the value for K for this reaction at 610K.
H2O2(g) is 2.3 × 106 at 610K. Determine the value for K for this reaction at 610K.A) 1.2 × 1010
B) 4.6 × 104
C) 1.2 × 108
D) 2.3 × 106
E) 4.3 × 10-7

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
13
For the reaction 2H2(g) + O2(g)  2H2O(g), what is the relationship between K and Kp at temperature T?
2H2O(g), what is the relationship between K and Kp at temperature T?
A) Kp = K(RT)2
B) K = Kp(RT)
C) K = Kp
D) Kp = K(RT)
E) K = Kp(RT)2
 2H2O(g), what is the relationship between K and Kp at temperature T?
2H2O(g), what is the relationship between K and Kp at temperature T?A) Kp = K(RT)2
B) K = Kp(RT)
C) K = Kp
D) Kp = K(RT)
E) K = Kp(RT)2

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
14
At -90°C, K for the reaction
N2O4(g) 2NO2(g)
2NO2(g)
Is 4.66 × 10-8. We introduce 0.038 mol of N2O4 into a 2.3-L vessel at -90°C and let equilibrium be established. The total pressure in the system at equilibrium will be
A) 4.66 × 10-8 atm.
B) 0.25 atm.
C) 0.12 atm.
D) 0.50 atm.
E) 0.23 atm.
N2O4(g)
 2NO2(g)
2NO2(g)Is 4.66 × 10-8. We introduce 0.038 mol of N2O4 into a 2.3-L vessel at -90°C and let equilibrium be established. The total pressure in the system at equilibrium will be
A) 4.66 × 10-8 atm.
B) 0.25 atm.
C) 0.12 atm.
D) 0.50 atm.
E) 0.23 atm.

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
15
Indicate the mass action expression for the following reaction:
2X(g) + Y(g)![<strong>Indicate the mass action expression for the following reaction: 2X(g) + Y(g) 3W(g) + V(g)</strong> A) B) C) [X]<sup>2</sup>[Y][W]<sup>3</sup>[V] D)](https://d2lvgg3v3hfg70.cloudfront.net/TB6422/11eaaf91_9e30_5e4d_892c_fbcfb08f7dbe_TB6422_11.jpg)
3W(g) + V(g)
A)![<strong>Indicate the mass action expression for the following reaction: 2X(g) + Y(g) 3W(g) + V(g)</strong> A) B) C) [X]<sup>2</sup>[Y][W]<sup>3</sup>[V] D)](https://d2lvgg3v3hfg70.cloudfront.net/TB6422/11eaaf91_9e30_5e4e_892c_d5bfd37747ed_TB6422_11.jpg)
B)![<strong>Indicate the mass action expression for the following reaction: 2X(g) + Y(g) 3W(g) + V(g)</strong> A) B) C) [X]<sup>2</sup>[Y][W]<sup>3</sup>[V] D)](https://d2lvgg3v3hfg70.cloudfront.net/TB6422/11eaaf91_9e30_855f_892c_2fb9a1753845_TB6422_11.jpg)
C) [X]2[Y][W]3[V]
D)![<strong>Indicate the mass action expression for the following reaction: 2X(g) + Y(g) 3W(g) + V(g)</strong> A) B) C) [X]<sup>2</sup>[Y][W]<sup>3</sup>[V] D)](https://d2lvgg3v3hfg70.cloudfront.net/TB6422/11eaaf91_9e30_8560_892c_d94013ec2390_TB6422_11.jpg)
2X(g) + Y(g)
![<strong>Indicate the mass action expression for the following reaction: 2X(g) + Y(g) 3W(g) + V(g)</strong> A) B) C) [X]<sup>2</sup>[Y][W]<sup>3</sup>[V] D)](https://d2lvgg3v3hfg70.cloudfront.net/TB6422/11eaaf91_9e30_5e4d_892c_fbcfb08f7dbe_TB6422_11.jpg)
3W(g) + V(g)
A)
![<strong>Indicate the mass action expression for the following reaction: 2X(g) + Y(g) 3W(g) + V(g)</strong> A) B) C) [X]<sup>2</sup>[Y][W]<sup>3</sup>[V] D)](https://d2lvgg3v3hfg70.cloudfront.net/TB6422/11eaaf91_9e30_5e4e_892c_d5bfd37747ed_TB6422_11.jpg)
B)
![<strong>Indicate the mass action expression for the following reaction: 2X(g) + Y(g) 3W(g) + V(g)</strong> A) B) C) [X]<sup>2</sup>[Y][W]<sup>3</sup>[V] D)](https://d2lvgg3v3hfg70.cloudfront.net/TB6422/11eaaf91_9e30_855f_892c_2fb9a1753845_TB6422_11.jpg)
C) [X]2[Y][W]3[V]
D)
![<strong>Indicate the mass action expression for the following reaction: 2X(g) + Y(g) 3W(g) + V(g)</strong> A) B) C) [X]<sup>2</sup>[Y][W]<sup>3</sup>[V] D)](https://d2lvgg3v3hfg70.cloudfront.net/TB6422/11eaaf91_9e30_8560_892c_d94013ec2390_TB6422_11.jpg)

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
16
For the hypothetical reactions 1 and 2, K1 = 102 and K2 = 10-4.
1) A2(g) + B2(g) 2AB(g)
2AB(g)
2) 2A2(g) + C2(g) 2A2C(g)
2A2C(g)
3) A2C(g) + B2(g) 2AB(g) + (1/2)C2(g)
2AB(g) + (1/2)C2(g)
What is the value for K for reaction 3?
A) 10-2
B) 104
C) 106
D) 102
E) 10-4
1) A2(g) + B2(g)
 2AB(g)
2AB(g)2) 2A2(g) + C2(g)
 2A2C(g)
2A2C(g)3) A2C(g) + B2(g)
 2AB(g) + (1/2)C2(g)
2AB(g) + (1/2)C2(g)What is the value for K for reaction 3?
A) 10-2
B) 104
C) 106
D) 102
E) 10-4

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
17
The equilibrium constant for A + 2B  3C is 1.0 × 10-6.Determine the equilibrium constant for 4A +8B
3C is 1.0 × 10-6.Determine the equilibrium constant for 4A +8B  12C
12C
A) 1.0 × 10-24
B) 1.0 × 1024
C) 1.0 × 10-6
D) 4 × 10-24
E) 4× 10-6
 3C is 1.0 × 10-6.Determine the equilibrium constant for 4A +8B
3C is 1.0 × 10-6.Determine the equilibrium constant for 4A +8B  12C
12CA) 1.0 × 10-24
B) 1.0 × 1024
C) 1.0 × 10-6
D) 4 × 10-24
E) 4× 10-6

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
18
The reaction
H2(g) + I2(g) 2HI(g)
2HI(g)
Has Kp = 45.9 at 763 K. A particular equilibrium mixture at that temperature contains gaseous HI at a partial pressure of 4.46 atm and hydrogen gas at a partial pressure of 0.240 atm. What is the partial pressure of I2?
A) 1.81 atm
B) 0.240 atm
C) 0.810 atm
D) 36.7 atm
E) 0.405 atm
H2(g) + I2(g)
 2HI(g)
2HI(g)Has Kp = 45.9 at 763 K. A particular equilibrium mixture at that temperature contains gaseous HI at a partial pressure of 4.46 atm and hydrogen gas at a partial pressure of 0.240 atm. What is the partial pressure of I2?
A) 1.81 atm
B) 0.240 atm
C) 0.810 atm
D) 36.7 atm
E) 0.405 atm

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
19
If, at a given temperature, the equilibrium constant for the reaction
H2(g) + Cl2(g) ⇌ 2HCl(g) is 5.0, then the equilibrium constant for the reaction
HCl(g) ⇌ (1/2)H2(g) + (1/2)Cl2(g) can be represented as
A)0.040.
B) 25.
C) 0.45.
D) 0.20.
E) 5.0.
H2(g) + Cl2(g) ⇌ 2HCl(g) is 5.0, then the equilibrium constant for the reaction
HCl(g) ⇌ (1/2)H2(g) + (1/2)Cl2(g) can be represented as
A)0.040.
B) 25.
C) 0.45.
D) 0.20.
E) 5.0.

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
20
A system at a state of chemical equilibrium is
A) microscopically dynamic and macroscopically dynamic.
B) microscopically dynamic and macroscopically static.
C) microscopically static and macroscopically static.
D) microscopically static and macroscopically dynamic.
E) none of these
A) microscopically dynamic and macroscopically dynamic.
B) microscopically dynamic and macroscopically static.
C) microscopically static and macroscopically static.
D) microscopically static and macroscopically dynamic.
E) none of these

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
21
Consider the following reaction:
2HF(g)
H2(g) + F2(g) (K = 1.00 × 10-2)
Given 1.18 mol of HF(g), 0.880 mol of H2(g), and 1.03 mol of F2(g) are mixed in a 3.00-L flask, determine the reaction quotient, Q, and the net direction to achieve equilibrium.
A) Q = 0.651; the equilibrium shifts to the right.
B) Q =0.768; the equilibrium shifts to the left.
C) Q = 0.768; the equilibrium shifts to the right.
D) Q = 0.651; the equilibrium shifts to the left.
E) Q = 1.17; the system is at equilibrium.
2HF(g)

H2(g) + F2(g) (K = 1.00 × 10-2)
Given 1.18 mol of HF(g), 0.880 mol of H2(g), and 1.03 mol of F2(g) are mixed in a 3.00-L flask, determine the reaction quotient, Q, and the net direction to achieve equilibrium.
A) Q = 0.651; the equilibrium shifts to the right.
B) Q =0.768; the equilibrium shifts to the left.
C) Q = 0.768; the equilibrium shifts to the right.
D) Q = 0.651; the equilibrium shifts to the left.
E) Q = 1.17; the system is at equilibrium.

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
22
At a given temperature, the equilibrium constant K for the reaction
2SO2(g) + O2(g)
2SO3(g)
Is 3.0 × 109. If 2.64 mol of SO2 and 2.98 mol of O2 are placed in a 1.77-L container and allowed to react to equilibrium at this temperature, what is the concentration of SO3 at equilibrium?
A) 1.49 M
B) 0.192 M
C) 2.98 M
D) 3.18 M
E) 1.68M
2SO2(g) + O2(g)

2SO3(g)
Is 3.0 × 109. If 2.64 mol of SO2 and 2.98 mol of O2 are placed in a 1.77-L container and allowed to react to equilibrium at this temperature, what is the concentration of SO3 at equilibrium?
A) 1.49 M
B) 0.192 M
C) 2.98 M
D) 3.18 M
E) 1.68M

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
23
At a certain temperature, K for the reaction
2NO2
N2O4
is 7.5 L/mol. If 2.0 mol of NO2 is placed in a 2.0-liter container and permitted to react at this temperature, calculate the concentration of N2O4 at equilibrium.
A) 0.39 mol/L
B) 0.82 mol/L
C) 7.5 mol/L
D) 0.65 mol/L
E) none of these
2NO2

N2O4
is 7.5 L/mol. If 2.0 mol of NO2 is placed in a 2.0-liter container and permitted to react at this temperature, calculate the concentration of N2O4 at equilibrium.
A) 0.39 mol/L
B) 0.82 mol/L
C) 7.5 mol/L
D) 0.65 mol/L
E) none of these

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
24
Consider the reaction
2NOBr(g)
2NO(g) + Br2(g)
A 1)0-L vessel was initially filled with pure NOBr at a pressure of 3.8 atm and 300 K. At equilibrium, the partial pressure of NOBr was 2.2 atm. Determine the value of Kp for the reaction.
A) 1.2
B) 2.4
C) 0.58
D) 0.42
E) 0.73
2NOBr(g)

2NO(g) + Br2(g)
A 1)0-L vessel was initially filled with pure NOBr at a pressure of 3.8 atm and 300 K. At equilibrium, the partial pressure of NOBr was 2.2 atm. Determine the value of Kp for the reaction.
A) 1.2
B) 2.4
C) 0.58
D) 0.42
E) 0.73

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
25
Consider the following reaction:
2NOCl(g)
2NO(g) + Cl2(g)
Initially pure NOCl(g) is placed in a vessel at 2.96 atm. At equilibrium, 0.410% of the NOCl has decomposed. Determine the value for Kp.
A) 5.00 × 10-5
B) 2.57 × 10-8
C) 6.07 × 10-3
D) 1.03 × 10-7
E) 2.06 × 10-7
2NOCl(g)

2NO(g) + Cl2(g)
Initially pure NOCl(g) is placed in a vessel at 2.96 atm. At equilibrium, 0.410% of the NOCl has decomposed. Determine the value for Kp.
A) 5.00 × 10-5
B) 2.57 × 10-8
C) 6.07 × 10-3
D) 1.03 × 10-7
E) 2.06 × 10-7

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
26
Consider the decomposition of hydrazine as shown below.N2H4(g)  2H2(g) + N2(g)
2H2(g) + N2(g)
At a certain temperature, Kp = 2.5 × 103. When pure hydrazine is placed in an otherwise empty vessel at this temperature, equilibrium is reached after 30.0% of the hydrazine has decomposed. Calculate the partial pressure of hydrogen gas at equilibrium.
A) 76 atm
B) 5776 atm
C) 54 atm
D) 127 atm
E) none of these
 2H2(g) + N2(g)
2H2(g) + N2(g)At a certain temperature, Kp = 2.5 × 103. When pure hydrazine is placed in an otherwise empty vessel at this temperature, equilibrium is reached after 30.0% of the hydrazine has decomposed. Calculate the partial pressure of hydrogen gas at equilibrium.
A) 76 atm
B) 5776 atm
C) 54 atm
D) 127 atm
E) none of these

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
27
A sample of solid NH4NO3 was placed in an evacuated container and then heated so that it decomposed explosively according to the following reaction:
NH4NO3(s)
N2O(g) + 2H2O(g)
At equilibrium, the total pressure in the container was found to be 2.77 atm at a temperature of 500°C. Calculate Kp.
A) 2.77
B) 7.67
C) 85.0
D) 3.15
E) 3.41
NH4NO3(s)

N2O(g) + 2H2O(g)
At equilibrium, the total pressure in the container was found to be 2.77 atm at a temperature of 500°C. Calculate Kp.
A) 2.77
B) 7.67
C) 85.0
D) 3.15
E) 3.41

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
28
Consider the equation 2A(g)  2B(g) + C(g). At a particular temperature, K = 1.6 × 104.If you start with 2.0 M of chemical A, calculate the equilibrium concentration of chemical C.
2B(g) + C(g). At a particular temperature, K = 1.6 × 104.If you start with 2.0 M of chemical A, calculate the equilibrium concentration of chemical C.
A) 8.3 × 10-3 M
B) 1.6 × 10-2 M
C) 6.25 × 10-5 M
D) 2.0 M
E) 0.98 M
 2B(g) + C(g). At a particular temperature, K = 1.6 × 104.If you start with 2.0 M of chemical A, calculate the equilibrium concentration of chemical C.
2B(g) + C(g). At a particular temperature, K = 1.6 × 104.If you start with 2.0 M of chemical A, calculate the equilibrium concentration of chemical C.A) 8.3 × 10-3 M
B) 1.6 × 10-2 M
C) 6.25 × 10-5 M
D) 2.0 M
E) 0.98 M

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
29
Nitric oxide, an important pollutant in air, is formed from the elements nitrogen and oxygen at high temperatures, such as those obtained when gasoline burns in an automobile engine. At 2000°C, K for the reaction N2(g) + O2(g)  2NO(g) is 0.01.Predict the direction in which the system will move to reach equilibrium at 2000°C if 0.4 mol of N2, 0.1 mol of O2, and 0.08 mol of NO are placed in a 1.0-L container.
2NO(g) is 0.01.Predict the direction in which the system will move to reach equilibrium at 2000°C if 0.4 mol of N2, 0.1 mol of O2, and 0.08 mol of NO are placed in a 1.0-L container.
A) The system remains unchanged.
B) The concentration of NO will decrease; the concentrations of N2 and O2 will remain unchanged.
C) The concentration of NO will decrease; the concentrations of N2 and O2 will increase.
D) The concentration of NO will increase; the concentrations of N2 and O2 will decrease.
E) More information is necessary.
 2NO(g) is 0.01.Predict the direction in which the system will move to reach equilibrium at 2000°C if 0.4 mol of N2, 0.1 mol of O2, and 0.08 mol of NO are placed in a 1.0-L container.
2NO(g) is 0.01.Predict the direction in which the system will move to reach equilibrium at 2000°C if 0.4 mol of N2, 0.1 mol of O2, and 0.08 mol of NO are placed in a 1.0-L container.A) The system remains unchanged.
B) The concentration of NO will decrease; the concentrations of N2 and O2 will remain unchanged.
C) The concentration of NO will decrease; the concentrations of N2 and O2 will increase.
D) The concentration of NO will increase; the concentrations of N2 and O2 will decrease.
E) More information is necessary.

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
30
Consider the equation 2A(g)  2B(g) + C(g). At a particular temperature, K = 1.6 × 104.At a higher temperature, K = 1.8 × 10-5. If you start with 2.0 M of chemical A, calculate the equilibrium concentration of chemical C.
2B(g) + C(g). At a particular temperature, K = 1.6 × 104.At a higher temperature, K = 1.8 × 10-5. If you start with 2.0 M of chemical A, calculate the equilibrium concentration of chemical C.
A) 2.6 × 10-2 M
B) 6.0 × 10-3 M
C) 1.0 M
D) 2.1 × 10-2 M
E) none of these
 2B(g) + C(g). At a particular temperature, K = 1.6 × 104.At a higher temperature, K = 1.8 × 10-5. If you start with 2.0 M of chemical A, calculate the equilibrium concentration of chemical C.
2B(g) + C(g). At a particular temperature, K = 1.6 × 104.At a higher temperature, K = 1.8 × 10-5. If you start with 2.0 M of chemical A, calculate the equilibrium concentration of chemical C.A) 2.6 × 10-2 M
B) 6.0 × 10-3 M
C) 1.0 M
D) 2.1 × 10-2 M
E) none of these

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
31
Which of the following is an example of a homogeneous equilibrium?
A) CaCl2(s) + 2H2O(g) CaCl2•2H2O(s)
CaCl2•2H2O(s)
B) H2(g) + I2(s) 2HI(g)
2HI(g)
C) NH4NO3(s) N2O(g) + 2H2O(g)
N2O(g) + 2H2O(g)
D) 2N2O(g) + N2H4(g) 3N2(g) + 2H2O(g)
3N2(g) + 2H2O(g)
E) none of these
A) CaCl2(s) + 2H2O(g)
 CaCl2•2H2O(s)
CaCl2•2H2O(s)B) H2(g) + I2(s)
 2HI(g)
2HI(g)C) NH4NO3(s)
 N2O(g) + 2H2O(g)
N2O(g) + 2H2O(g)D) 2N2O(g) + N2H4(g)
 3N2(g) + 2H2O(g)
3N2(g) + 2H2O(g)E) none of these

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
32
What is the equilibrium expression for the following reaction?
NH4NO3(s)![<strong>What is the equilibrium expression for the following reaction? NH<sub>4</sub>NO<sub>3</sub>(s) N<sub>2</sub>O(g) + 2H<sub>2</sub>O(g)</strong> A) B) C) D) [N<sub>2</sub>O][H<sub>2</sub>O]<sup>2</sup> E) none of these](https://d2lvgg3v3hfg70.cloudfront.net/TB6422/11eaaf91_9e32_3333_892c_8fd8d2109b40_TB6422_11.jpg)
N2O(g) + 2H2O(g)
A)![<strong>What is the equilibrium expression for the following reaction? NH<sub>4</sub>NO<sub>3</sub>(s) N<sub>2</sub>O(g) + 2H<sub>2</sub>O(g)</strong> A) B) C) D) [N<sub>2</sub>O][H<sub>2</sub>O]<sup>2</sup> E) none of these](https://d2lvgg3v3hfg70.cloudfront.net/TB6422/11eaaf91_9e32_5a44_892c_31f8553f95c8_TB6422_11.jpg)
B)![<strong>What is the equilibrium expression for the following reaction? NH<sub>4</sub>NO<sub>3</sub>(s) N<sub>2</sub>O(g) + 2H<sub>2</sub>O(g)</strong> A) B) C) D) [N<sub>2</sub>O][H<sub>2</sub>O]<sup>2</sup> E) none of these](https://d2lvgg3v3hfg70.cloudfront.net/TB6422/11eaaf91_9e32_5a45_892c_d753c81258b1_TB6422_11.jpg)
C)![<strong>What is the equilibrium expression for the following reaction? NH<sub>4</sub>NO<sub>3</sub>(s) N<sub>2</sub>O(g) + 2H<sub>2</sub>O(g)</strong> A) B) C) D) [N<sub>2</sub>O][H<sub>2</sub>O]<sup>2</sup> E) none of these](https://d2lvgg3v3hfg70.cloudfront.net/TB6422/11eaaf91_9e32_5a46_892c_8fb4f994b33d_TB6422_11.jpg)
D) [N2O][H2O]2
E) none of these
NH4NO3(s)
![<strong>What is the equilibrium expression for the following reaction? NH<sub>4</sub>NO<sub>3</sub>(s) N<sub>2</sub>O(g) + 2H<sub>2</sub>O(g)</strong> A) B) C) D) [N<sub>2</sub>O][H<sub>2</sub>O]<sup>2</sup> E) none of these](https://d2lvgg3v3hfg70.cloudfront.net/TB6422/11eaaf91_9e32_3333_892c_8fd8d2109b40_TB6422_11.jpg)
N2O(g) + 2H2O(g)
A)
![<strong>What is the equilibrium expression for the following reaction? NH<sub>4</sub>NO<sub>3</sub>(s) N<sub>2</sub>O(g) + 2H<sub>2</sub>O(g)</strong> A) B) C) D) [N<sub>2</sub>O][H<sub>2</sub>O]<sup>2</sup> E) none of these](https://d2lvgg3v3hfg70.cloudfront.net/TB6422/11eaaf91_9e32_5a44_892c_31f8553f95c8_TB6422_11.jpg)
B)
![<strong>What is the equilibrium expression for the following reaction? NH<sub>4</sub>NO<sub>3</sub>(s) N<sub>2</sub>O(g) + 2H<sub>2</sub>O(g)</strong> A) B) C) D) [N<sub>2</sub>O][H<sub>2</sub>O]<sup>2</sup> E) none of these](https://d2lvgg3v3hfg70.cloudfront.net/TB6422/11eaaf91_9e32_5a45_892c_d753c81258b1_TB6422_11.jpg)
C)
![<strong>What is the equilibrium expression for the following reaction? NH<sub>4</sub>NO<sub>3</sub>(s) N<sub>2</sub>O(g) + 2H<sub>2</sub>O(g)</strong> A) B) C) D) [N<sub>2</sub>O][H<sub>2</sub>O]<sup>2</sup> E) none of these](https://d2lvgg3v3hfg70.cloudfront.net/TB6422/11eaaf91_9e32_5a46_892c_8fb4f994b33d_TB6422_11.jpg)
D) [N2O][H2O]2
E) none of these

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
33
The following reaction is investigated (assume an ideal gas mixture).2N2O(g) + N2H4(g)  3N2(g) + 2H2O(g)
3N2(g) + 2H2O(g)
Initially there are 0.08 mol of N2O and 0.38 mol of N2H4, in a 30.0-L container. If there is 0.050 mol of N2O at equilibrium, how many moles of N2 are present at equilibrium?
A) 0.053
B) 0.045
C) 0.12
D) 0.030
E) 0.15
 3N2(g) + 2H2O(g)
3N2(g) + 2H2O(g)Initially there are 0.08 mol of N2O and 0.38 mol of N2H4, in a 30.0-L container. If there is 0.050 mol of N2O at equilibrium, how many moles of N2 are present at equilibrium?
A) 0.053
B) 0.045
C) 0.12
D) 0.030
E) 0.15

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
34
Consider the following reaction (assume an ideal gas mixture).2NOBr(g)  2NO(g) + Br2(g)
2NO(g) + Br2(g)
A 3)0-L vessel was initially filled with pure NOBr, at a pressure of 3.9 atm, at 310K.After equilibrium was established, the partial pressure of NOBr was 2.1 atm. What is Kp for the reaction?
A) 0.66
B) 0.22
C) 1.32
D) 1.54
E) 1.1
 2NO(g) + Br2(g)
2NO(g) + Br2(g)A 3)0-L vessel was initially filled with pure NOBr, at a pressure of 3.9 atm, at 310K.After equilibrium was established, the partial pressure of NOBr was 2.1 atm. What is Kp for the reaction?
A) 0.66
B) 0.22
C) 1.32
D) 1.54
E) 1.1

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
35
Nitric oxide, an important pollutant in air, is formed from the elements nitrogen and oxygen at high temperatures, such as those obtained when gasoline burns in an automobile engine. At 2000°C, K for the reaction N2(g) + O2(g)  2NO(g) is 0.01.A 1-L container originally holds 0.4 mol of N2, 0.1 mol of O2, and 0.08 mol of NO. If the volume of the container holding the equilibrium mixture of N2, O2, and NO is decreased to 0.5 L without changing the quantities of the gases present, how will their concentrations change?
2NO(g) is 0.01.A 1-L container originally holds 0.4 mol of N2, 0.1 mol of O2, and 0.08 mol of NO. If the volume of the container holding the equilibrium mixture of N2, O2, and NO is decreased to 0.5 L without changing the quantities of the gases present, how will their concentrations change?
A) The concentrations of N2 and O2 will increase, and the concentration of NO will decrease.
B) The concentrations of N2, O2, and NO will decrease.
C) The concentrations of N2, O2, and NO will increase.
D) There will be no change in the concentrations of N2, O2, and NO.
E) The concentration of NO will increase, and the concentrations of N2 and O2 will decrease.
 2NO(g) is 0.01.A 1-L container originally holds 0.4 mol of N2, 0.1 mol of O2, and 0.08 mol of NO. If the volume of the container holding the equilibrium mixture of N2, O2, and NO is decreased to 0.5 L without changing the quantities of the gases present, how will their concentrations change?
2NO(g) is 0.01.A 1-L container originally holds 0.4 mol of N2, 0.1 mol of O2, and 0.08 mol of NO. If the volume of the container holding the equilibrium mixture of N2, O2, and NO is decreased to 0.5 L without changing the quantities of the gases present, how will their concentrations change?A) The concentrations of N2 and O2 will increase, and the concentration of NO will decrease.
B) The concentrations of N2, O2, and NO will decrease.
C) The concentrations of N2, O2, and NO will increase.
D) There will be no change in the concentrations of N2, O2, and NO.
E) The concentration of NO will increase, and the concentrations of N2 and O2 will decrease.

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
36
For a particular system at a particular temperature, there are ______ equilibrium constant(s) and _______ equilibrium position(s).
A) one, one
B) one, an infinite number of
C) an infinite number of, one
D) an infinite number of, an infinite number of
E) none of these
A) one, one
B) one, an infinite number of
C) an infinite number of, one
D) an infinite number of, an infinite number of
E) none of these

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
37
Consider the following equilibrium:
N2(g) + 3H2(g)
2NH3(g)
With K = 2.3 × 10-6. 1.00 mol each of all reactants and products is placed in a 1.00-L container.Which way will the reaction initially proceed?
A) To the right.
B) We need to know the temperature.
C) The system is at equilibrium.
D) To the left.
E) none of these
N2(g) + 3H2(g)

2NH3(g)
With K = 2.3 × 10-6. 1.00 mol each of all reactants and products is placed in a 1.00-L container.Which way will the reaction initially proceed?
A) To the right.
B) We need to know the temperature.
C) The system is at equilibrium.
D) To the left.
E) none of these

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
38
Consider the following equilibrium: 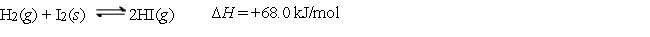 Which of the following is the proper Keq expression?
Which of the following is the proper Keq expression?
A)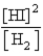
B)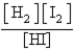
C)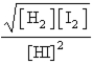
D)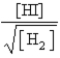
E)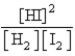
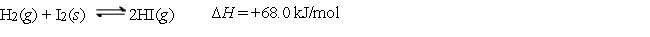 Which of the following is the proper Keq expression?
Which of the following is the proper Keq expression?A)
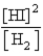
B)
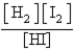
C)
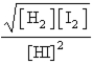
D)
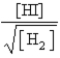
E)
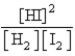

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
39
Consider the equation 2A(g)  2B(g) + C(g). At a particular temperature, K = 1.6 × 104.If you mixed 5.0 mol B, 0.10 mol C, and 0.0010 mol A in a 1-L container, in which direction would the reaction initially proceed?
2B(g) + C(g). At a particular temperature, K = 1.6 × 104.If you mixed 5.0 mol B, 0.10 mol C, and 0.0010 mol A in a 1-L container, in which direction would the reaction initially proceed?
A) To the right.
B) To the left.
C) The above mixture is the equilibrium mixture.
D) We cannot tell from the information given.
 2B(g) + C(g). At a particular temperature, K = 1.6 × 104.If you mixed 5.0 mol B, 0.10 mol C, and 0.0010 mol A in a 1-L container, in which direction would the reaction initially proceed?
2B(g) + C(g). At a particular temperature, K = 1.6 × 104.If you mixed 5.0 mol B, 0.10 mol C, and 0.0010 mol A in a 1-L container, in which direction would the reaction initially proceed?A) To the right.
B) To the left.
C) The above mixture is the equilibrium mixture.
D) We cannot tell from the information given.

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
40
Consider the reaction
2SO2(g) + O2(g)
2SO3(g)
At constant temperature. Initially a container is filled with pure SO3(g) at a pressure of 2 atm, after which equilibrium is allowed to be reached. If y is the partial pressure of O2 at equilibrium, what is the value of Kp?
A)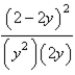
B)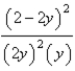
C)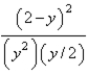
D)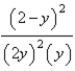
E) none of these
2SO2(g) + O2(g)

2SO3(g)
At constant temperature. Initially a container is filled with pure SO3(g) at a pressure of 2 atm, after which equilibrium is allowed to be reached. If y is the partial pressure of O2 at equilibrium, what is the value of Kp?
A)
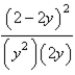
B)
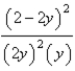
C)
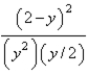
D)
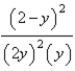
E) none of these

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
41
Nitrogen gas (N2) reacts with hydrogen gas (H2) to form ammonia (NH3). At 200°C in a closed container, 1.2atm of nitrogen gas is mixed with 2.3 atm of hydrogen gas. At equilibrium, the total pressure is 2.1 atm. Calculate the partial pressure of hydrogen gas at equilibrium.
A) 2.3 atm
B) 0.0 atm
C) 1.8 atm
D) 0.20 atm
E) 0.92 atm
A) 2.3 atm
B) 0.0 atm
C) 1.8 atm
D) 0.20 atm
E) 0.92 atm

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
42
Explain how a given system at a given temperature has one equilibrium constant but an infinite number of equilibrium positions.

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
43
Which of the following statements is false?
A) Decreasing the volume of the container shifts the equilibrium to form more PCl5.
B) Removing PCl5 from the container shifts the equilibrium to form more PCl3.
C) Adding PCl3 to the container shifts the equilibrium to form more PCl5.
D) Increasing the temperature shifts the equilibrium to form more PCl3.
A) Decreasing the volume of the container shifts the equilibrium to form more PCl5.
B) Removing PCl5 from the container shifts the equilibrium to form more PCl3.
C) Adding PCl3 to the container shifts the equilibrium to form more PCl5.
D) Increasing the temperature shifts the equilibrium to form more PCl3.

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
44
Raising the pressure by lowering the volume of the container will
A) cause [B] to increase.
B) have no effect.
C) cause [A] to increase.
D) cannot be determined
E) none of the these
A) cause [B] to increase.
B) have no effect.
C) cause [A] to increase.
D) cannot be determined
E) none of the these

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
45
Consider the following equilibrium: 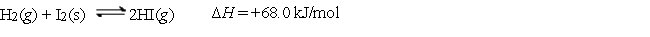 Which of the following statements about the equilibrium is false?
Which of the following statements about the equilibrium is false?
A) Removing HI as it forms forces the equilibrium to the right.
B) Adding more H2(g) increases the equilibrium constant.
C) This is a heterogeneous equilibrium.
D) If the pressure on the system is increased by changing the volume, the left side is favored.
E) If the system is heated, the right side is favored.
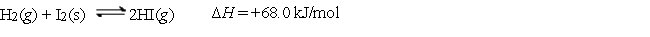 Which of the following statements about the equilibrium is false?
Which of the following statements about the equilibrium is false?A) Removing HI as it forms forces the equilibrium to the right.
B) Adding more H2(g) increases the equilibrium constant.
C) This is a heterogeneous equilibrium.
D) If the pressure on the system is increased by changing the volume, the left side is favored.
E) If the system is heated, the right side is favored.

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
46
Consider the following equilibrium:N2(g) + 3H2(g)  2NH3(g)
2NH3(g)
with K = 2.3 × 10-6. 1.00 mol each of all reactants and products is placed in a 1.00-L container.
Calculate the equilibrium concentration of N2.
A) 1.5 M
B) 2.0 M
C) 0.5 M
D) 2.5 M
E) 1.0 M
 2NH3(g)
2NH3(g)with K = 2.3 × 10-6. 1.00 mol each of all reactants and products is placed in a 1.00-L container.
Calculate the equilibrium concentration of N2.
A) 1.5 M
B) 2.0 M
C) 0.5 M
D) 2.5 M
E) 1.0 M

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
47
Consider the equation 2A(g) ![<strong>Consider the equation 2A(g) 2B(g) + C(g). At a particular temperature, K = 1.6 × 10<sup>4</sup>. Addition of chemical B to an equilibrium mixture of the above will</strong> A) cause [A] to increase. B) have no effect. C) cause [C] to increase. D) cannot be determined E) none of these](https://d2lvgg3v3hfg70.cloudfront.net/TB6422/11eaaf91_9e34_f272_892c_839d3578b933_TB6422_11.jpg) 2B(g) + C(g). At a particular temperature, K = 1.6 × 104.
2B(g) + C(g). At a particular temperature, K = 1.6 × 104.
Addition of chemical B to an equilibrium mixture of the above will
A) cause [A] to increase.
B) have no effect.
C) cause [C] to increase.
D) cannot be determined
E) none of these
![<strong>Consider the equation 2A(g) 2B(g) + C(g). At a particular temperature, K = 1.6 × 10<sup>4</sup>. Addition of chemical B to an equilibrium mixture of the above will</strong> A) cause [A] to increase. B) have no effect. C) cause [C] to increase. D) cannot be determined E) none of these](https://d2lvgg3v3hfg70.cloudfront.net/TB6422/11eaaf91_9e34_f272_892c_839d3578b933_TB6422_11.jpg) 2B(g) + C(g). At a particular temperature, K = 1.6 × 104.
2B(g) + C(g). At a particular temperature, K = 1.6 × 104.Addition of chemical B to an equilibrium mixture of the above will
A) cause [A] to increase.
B) have no effect.
C) cause [C] to increase.
D) cannot be determined
E) none of these

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
48
Consider the following equilibrium:N2(g) + 3H2(g)  2NH3(g)
2NH3(g)
with K = 2.3 × 10-6. 1.00 mol each of all reactants and products is placed in a 1.00-L container.
Calculate the equilibrium concentration of H2.
A) 0.5 M
B) 1.0 M
C) 2.5 M
D) 2.0 M
E) 1.5 M
 2NH3(g)
2NH3(g)with K = 2.3 × 10-6. 1.00 mol each of all reactants and products is placed in a 1.00-L container.
Calculate the equilibrium concentration of H2.
A) 0.5 M
B) 1.0 M
C) 2.5 M
D) 2.0 M
E) 1.5 M

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
49
Consider the following reaction: 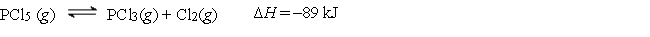
How can the equilibrium be shifted to the right?
A) Remove Cl2.
B) Decrease the pressure by changing the volume.
C) Remove PCl3.
D) Add more PCl5.
E) Any of these will shift the equilibrium to the right.
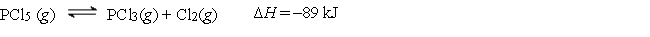
How can the equilibrium be shifted to the right?
A) Remove Cl2.
B) Decrease the pressure by changing the volume.
C) Remove PCl3.
D) Add more PCl5.
E) Any of these will shift the equilibrium to the right.

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
50
Consider the following equilibrium:N2(g) + 3H2(g)  2NH3(g)
2NH3(g)
with K = 2.3 × 10-6. 1.00 mol each of all reactants and products is placed in a 1.00-L container.
Calculate the equilibrium concentration of NH3(g).
A) 3.7 × 10-3 M
B) 5.4 × 10-5 M
C) 7.3 × 10-3 M
D) 4.3 × 10-6 M
E) none of these
 2NH3(g)
2NH3(g)with K = 2.3 × 10-6. 1.00 mol each of all reactants and products is placed in a 1.00-L container.
Calculate the equilibrium concentration of NH3(g).
A) 3.7 × 10-3 M
B) 5.4 × 10-5 M
C) 7.3 × 10-3 M
D) 4.3 × 10-6 M
E) none of these

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
51
To increase the value of K for the exothermic reaction
2H2(g) + O2(g)
H2O(g)
We should
A) decrease the temperature.
B) decrease the total pressure.
C) increase the total pressure.
D) increase the temperature.
E) Two of these are necessary.
2H2(g) + O2(g)

H2O(g)
We should
A) decrease the temperature.
B) decrease the total pressure.
C) increase the total pressure.
D) increase the temperature.
E) Two of these are necessary.

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
52
Consider the equation 2A(g) ![<strong>Consider the equation 2A(g) 2B(g) + C(g). At a particular temperature, K = 1.6 × 10<sup>4</sup>.Placing the equilibrium mixture in an ice bath (thus lowering the temperature) will</strong> A) have no effect. B) cause [A] to increase. C) cause [B] to increase. D) cannot be determined E) none of these](https://d2lvgg3v3hfg70.cloudfront.net/TB6422/11eaaf91_9e35_67a5_892c_5d4b1582d60b_TB6422_11.jpg) 2B(g) + C(g). At a particular temperature, K = 1.6 × 104.Placing the equilibrium mixture in an ice bath (thus lowering the temperature) will
2B(g) + C(g). At a particular temperature, K = 1.6 × 104.Placing the equilibrium mixture in an ice bath (thus lowering the temperature) will
A) have no effect.
B) cause [A] to increase.
C) cause [B] to increase.
D) cannot be determined
E) none of these
![<strong>Consider the equation 2A(g) 2B(g) + C(g). At a particular temperature, K = 1.6 × 10<sup>4</sup>.Placing the equilibrium mixture in an ice bath (thus lowering the temperature) will</strong> A) have no effect. B) cause [A] to increase. C) cause [B] to increase. D) cannot be determined E) none of these](https://d2lvgg3v3hfg70.cloudfront.net/TB6422/11eaaf91_9e35_67a5_892c_5d4b1582d60b_TB6422_11.jpg) 2B(g) + C(g). At a particular temperature, K = 1.6 × 104.Placing the equilibrium mixture in an ice bath (thus lowering the temperature) will
2B(g) + C(g). At a particular temperature, K = 1.6 × 104.Placing the equilibrium mixture in an ice bath (thus lowering the temperature) willA) have no effect.
B) cause [A] to increase.
C) cause [B] to increase.
D) cannot be determined
E) none of these

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
53
When the substances in the equation below are at equilibrium at pressure P and temperature T, how can the equilibrium be shifted to favor the products?
CuO(s) + H2(g)
Cu(s) + H2O(g)
Change in enthalpy = -2.0 kJ.
A) Increase the pressure by adding an inert gas such as nitrogen.
B) Allow some gas to escape at constant pressure and temperature.
C) Decrease the temperature.
D) Increase the pressure by means of a moving piston at constant temperature.
E) Add a catalyst.
CuO(s) + H2(g)

Cu(s) + H2O(g)
Change in enthalpy = -2.0 kJ.
A) Increase the pressure by adding an inert gas such as nitrogen.
B) Allow some gas to escape at constant pressure and temperature.
C) Decrease the temperature.
D) Increase the pressure by means of a moving piston at constant temperature.
E) Add a catalyst.

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
54
For a certain reaction at 25.0°C, the value of K is 1.2 × 10-3. At 50.0°C the value of K is 3.4 × 10-1. This means that the reaction is
A) endothermic
B) exothermic
C) We need more information.
A) endothermic
B) exothermic
C) We need more information.

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
55
Consider the equation A(aq) + 2B(aq)  3C(aq) + 2D(aq). 40.0 mL of 0.056 M A is mixed with 25.0 mL 0.108 M B. At equilibrium, the concentration of C is 0.0412 M. Calculate K.
3C(aq) + 2D(aq). 40.0 mL of 0.056 M A is mixed with 25.0 mL 0.108 M B. At equilibrium, the concentration of C is 0.0412 M. Calculate K.
A) 0.029
B) 2.6
C) 0.0020
D) 1.9 × 10-4
E) 0.013
 3C(aq) + 2D(aq). 40.0 mL of 0.056 M A is mixed with 25.0 mL 0.108 M B. At equilibrium, the concentration of C is 0.0412 M. Calculate K.
3C(aq) + 2D(aq). 40.0 mL of 0.056 M A is mixed with 25.0 mL 0.108 M B. At equilibrium, the concentration of C is 0.0412 M. Calculate K.A) 0.029
B) 2.6
C) 0.0020
D) 1.9 × 10-4
E) 0.013

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
56
Which of the following statements is true?
A) Increasing the temperature of a system at equilibrium always increases the amount of product.
B) Increasing the temperature of a system at equilibrium changes the value of the equilibrium constant.
C) Increasing the temperature of a system at equilibrium always decreases the amount of product.
D) Changing the temperature of a system at equilibrium does not affect the equilibrium position.
E) none of these
A) Increasing the temperature of a system at equilibrium always increases the amount of product.
B) Increasing the temperature of a system at equilibrium changes the value of the equilibrium constant.
C) Increasing the temperature of a system at equilibrium always decreases the amount of product.
D) Changing the temperature of a system at equilibrium does not affect the equilibrium position.
E) none of these

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
57
Ammonia is prepared industrially by the following reaction:
N2(g) + 3H2(g)
2NH3(g)
For the reaction, ΔH° = -92.2 kJ and K (at 25°C) = 4.0 × 108. When the temperature of the reaction is increased to 500°C, which of the following statements is true?
A) Product formation (at equilibrium) is not favored as the temperature is raised.
B) K for the reaction will be larger at 500°C than at 25°C.
C) The reaction of N2 with H2 to form ammonia is endothermic.
D) At equilibrium, more NH3 is present at 500°C than at 25°C.
E) None of these is true.
N2(g) + 3H2(g)

2NH3(g)
For the reaction, ΔH° = -92.2 kJ and K (at 25°C) = 4.0 × 108. When the temperature of the reaction is increased to 500°C, which of the following statements is true?
A) Product formation (at equilibrium) is not favored as the temperature is raised.
B) K for the reaction will be larger at 500°C than at 25°C.
C) The reaction of N2 with H2 to form ammonia is endothermic.
D) At equilibrium, more NH3 is present at 500°C than at 25°C.
E) None of these is true.

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
58
Explain how chemical equilibrium is microscopically dynamic and macroscopically static.

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
59
Consider the following reaction (assume an ideal gas mixture).2NOBr(g)  2NO(g) + Br2(g)
2NO(g) + Br2(g)
A 2)0-L vessel was initially filled with pure NOBr, at a pressure of 3.9 atm, at 310 K.After equilibrium was reached, the volume was increased to 2.0 L, while the temperature was kept at 300 K. This will result in
A) a decrease in Kp.
B) a shift in the equilibrium position to the right.
C) an increase in Kp.
D) a shift in the equilibrium position to the left.
E) none of these
 2NO(g) + Br2(g)
2NO(g) + Br2(g)A 2)0-L vessel was initially filled with pure NOBr, at a pressure of 3.9 atm, at 310 K.After equilibrium was reached, the volume was increased to 2.0 L, while the temperature was kept at 300 K. This will result in
A) a decrease in Kp.
B) a shift in the equilibrium position to the right.
C) an increase in Kp.
D) a shift in the equilibrium position to the left.
E) none of these

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
60
Given the reaction A(g) + B(g)  C(g) + D(g). You have the gases A, B, C, and D at equilibrium. Upon adding gas A, the value of K
C(g) + D(g). You have the gases A, B, C, and D at equilibrium. Upon adding gas A, the value of K
A) depends on whether the reaction is endothermic or exothermic.
B) does not change as long as the temperature is constant.
C) does not change because A does not figure in the ratio of product to reactant.
D) decreases because A is a reactant, so the ratio of product to reactant decreases.
E) increases because when A is added, more products are made, increasing the ratio of product to reactant.
 C(g) + D(g). You have the gases A, B, C, and D at equilibrium. Upon adding gas A, the value of K
C(g) + D(g). You have the gases A, B, C, and D at equilibrium. Upon adding gas A, the value of KA) depends on whether the reaction is endothermic or exothermic.
B) does not change as long as the temperature is constant.
C) does not change because A does not figure in the ratio of product to reactant.
D) decreases because A is a reactant, so the ratio of product to reactant decreases.
E) increases because when A is added, more products are made, increasing the ratio of product to reactant.

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
61
To achieve equilibrium, the original reaction mixture will
A) produce more water and oxygen only
B) experience no change in component pressures
C) always move in a direction to lower the total pressure
D) shift toward products
E) shift toward reactants
A) produce more water and oxygen only
B) experience no change in component pressures
C) always move in a direction to lower the total pressure
D) shift toward products
E) shift toward reactants

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
62
Derive the relationship between K and Kp.

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
63
Consider the following chemical reaction involving a pure solid sample A that yields products B and C:
3A(s)
3B(g) + 2C(g)
Identify the equilibrium expression of the reaction.
A) 
B) 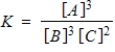
C) 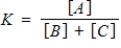
D) 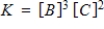
E) 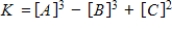
3A(s)

3B(g) + 2C(g)
Identify the equilibrium expression of the reaction.
A)

B)
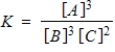
C)
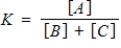
D)
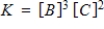
E)
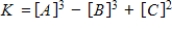

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
64
At room temperature cyclohexane exists almost exclusively in the chair conformation (99.99%). But at 800°C, 30% of the cyclohexane molecules exist in the twist-boat conformation.What is the value of the equilibrium constant for the following reaction at 800°C?
C6H12(chair)
C6H12(twist-boat)
A) 0.30
B) 2.3
C) 0.23
D) 0.43
E) 0.77
C6H12(chair)
C6H12(twist-boat)
A) 0.30
B) 2.3
C) 0.23
D) 0.43
E) 0.77

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
65
Which of the following determines the equilibrium position of a chemical reaction?
1) Initial concentration of the reactants
2) Relative energies of the reactants and products
3) Relative degree of organization of the reactants and products
A) 1 only
B) 1, 2, and 3
C) 2 only
D) 3 only
E) 1 and 3
1) Initial concentration of the reactants
2) Relative energies of the reactants and products
3) Relative degree of organization of the reactants and products
A) 1 only
B) 1, 2, and 3
C) 2 only
D) 3 only
E) 1 and 3

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
66
Write the equilibrium constant expression for the following reaction:
CaCO3(s)
CaO(s) + CO2(g)
CaCO3(s)

CaO(s) + CO2(g)

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
67
Once equilibrium was established, some additional chlorine gas was added to the system. This resulted in
A) no net change in the amounts of the other reaction components.
B) an increase in the amount of H2O relative to the equilibrium mixture.
C) a decrease in the amount of H2O relative to the equilibrium mixture.
D) a decrease in the amount of HCl relative to the equilibrium mixture.
E) both B and D
A) no net change in the amounts of the other reaction components.
B) an increase in the amount of H2O relative to the equilibrium mixture.
C) a decrease in the amount of H2O relative to the equilibrium mixture.
D) a decrease in the amount of HCl relative to the equilibrium mixture.
E) both B and D

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
68
For a chemical reaction system, Q and K are the reaction quotient and equilibrium constant, respectively. Identify the true statement(s).1. If Q is equal to K, the system is at equilibrium.2. If Q is greater than K, the ratio of initial concentrations of products to initial concentration of reactants is small.3. If Q is less than K, the ratio of initial concentration of products to initial concentration of reactants is large.
A) 1 only
B) 2 only
C) 3 only
D) 1 and 2
E) 2 and 3
A) 1 only
B) 2 only
C) 3 only
D) 1 and 2
E) 2 and 3

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
69
State Le Châtelier's principle.

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
70
At 25° C a sample of N2O4(g) is placed in an empty cylinder. After equilibrium is reached, the total pressure is 1.5 atm, and 16% (by moles) of the original N2O4 has dissociated to NO2(g). If the volume of the cylinder is increased until the total pressure is 1.0 atm, what percent (by moles) of the original N2O4(g) has dissociated at the new equilibrium position? (Hint: First calculate Kp.)

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
71
In the reaction
P4(g) 2P2(g)Kp = 0.500 atm at 1052°C.
2P2(g)Kp = 0.500 atm at 1052°C.
In an experiment, P4(g) is initially placed into a container at 1052°C. The total pressure of the equilibrium mixture of P4(g) and P2(g) is 6.00 atm. Calculate the equilibrium pressures of P4(g) and P2(g). Calculate the fraction (by moles) of P4(g) that has dissociated to reach equilibrium.
P4(g)
 2P2(g)Kp = 0.500 atm at 1052°C.
2P2(g)Kp = 0.500 atm at 1052°C.In an experiment, P4(g) is initially placed into a container at 1052°C. The total pressure of the equilibrium mixture of P4(g) and P2(g) is 6.00 atm. Calculate the equilibrium pressures of P4(g) and P2(g). Calculate the fraction (by moles) of P4(g) that has dissociated to reach equilibrium.

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
72
What is the partial pressure of O2 at equilibrium?
A) 2.18 atm
B) 0.91 atm
C) 1.64 atm
D) 1.18 atm
E) 2.27 atm
A) 2.18 atm
B) 0.91 atm
C) 1.64 atm
D) 1.18 atm
E) 2.27 atm

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
73
Identify the true statement(s) about equilibria.1. Homogeneous equilibria is the equilibria of systems in the gas phase.2. Heterogeneous equilibria is the equilibria of systems involving more than one phase.3. The concentration of pure solids and liquids involved in a chemical reaction is not included in the equilibrium expression for a given reaction.
A) 1 only
B) 2 only
C) 3 only
D) 1, 2, and 3
E) 2 and 3
A) 1 only
B) 2 only
C) 3 only
D) 1, 2, and 3
E) 2 and 3

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
74
Consider the following reaction, which is involved in the catalytic destruction of ozone by chlorine atoms.
ClO(g) + O3(g) Cl(g) + 2O2(g)
Cl(g) + 2O2(g)
Kp = 2.5 × 106 atm at 25° C. In an experiment at 25° C, ClO(g) at 1.0 × 10-3 atm was mixed with O3(g) at 2.0 ×10-5 atm in a rigid vessel. Calculate the equilibrium pressure of O3(g) and O2(g).
ClO(g) + O3(g)
 Cl(g) + 2O2(g)
Cl(g) + 2O2(g)Kp = 2.5 × 106 atm at 25° C. In an experiment at 25° C, ClO(g) at 1.0 × 10-3 atm was mixed with O3(g) at 2.0 ×10-5 atm in a rigid vessel. Calculate the equilibrium pressure of O3(g) and O2(g).

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
75
At equilibrium, the partial pressure of HCl will be
A) impossible to determine with this information
B) less than 2 atm
C) zero
D) between 2 and 4 atm
E) more than 4 atm
A) impossible to determine with this information
B) less than 2 atm
C) zero
D) between 2 and 4 atm
E) more than 4 atm

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
76
A mixture of O2(g) and O3(g) is present at equilibrium in a rigid container at 152 torr and 125° C. The density of the gaseous mixture is 0.228 g/L. Calculate Kp at 125° C for the reaction
3O2(g)
2O3(g)
3O2(g)

2O3(g)

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
77
The ratio of the equilibrium pressure (or concentration) for a given substance to a reference pressure (or concentration) for that substance is called the _____ of the substance.
A) molarity
B) activity
C) density
D) acclivity
E) affinity
A) molarity
B) activity
C) density
D) acclivity
E) affinity

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
78
What is the value of Q0, the reaction quotient, based on the conditions above?
A) 0.50
B) 0.125
C) 4.00
D) 1.00
E) None of the above
A) 0.50
B) 0.125
C) 4.00
D) 1.00
E) None of the above

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck



