Deck 14: Aldehydes, Ketones, and Chiral Molecules
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/83
Play
Full screen (f)
Deck 14: Aldehydes, Ketones, and Chiral Molecules
1
Formaldehyde is used industrially to make
A) polymers.
B) insulating materials.
C) carpeting.
D) All of the above.
E) None of the above.
A) polymers.
B) insulating materials.
C) carpeting.
D) All of the above.
E) None of the above.
All of the above.
2
What is the IUPAC name for this compound? 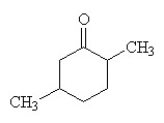
A) methylcyclohexanone
B) 2,5-dimethylcyclohexanone
C) 1,4-dimethyl-2-cyclohexanone
D) cyclohexyl methyl ketone
E) 1,4-dimethyl-3-cyclohexanone

A) methylcyclohexanone
B) 2,5-dimethylcyclohexanone
C) 1,4-dimethyl-2-cyclohexanone
D) cyclohexyl methyl ketone
E) 1,4-dimethyl-3-cyclohexanone
2,5-dimethylcyclohexanone
3
How many lone pairs of electrons does the oxygen in a carbonyl group have?
A) None, they're all bonded.
B) one
C) two
D) three
E) four
A) None, they're all bonded.
B) one
C) two
D) three
E) four
two
4
What are the bond angles in a typical carbonyl group?
A) 45°
B) 90°
C) 109.5°
D) 120°
E) 135°
A) 45°
B) 90°
C) 109.5°
D) 120°
E) 135°

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
5
How many hydrogen atoms is the carbonyl group in a ketone bonded to?
A) none
B) one
C) two
D) three
E) four
A) none
B) one
C) two
D) three
E) four

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
6
Which one of the following compounds is an aldehyde?
A)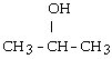
B)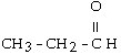
C) CH3 - CH= CH - CH₂ - CH3
D) CH3 - CH₂ - O - CH₂ - CH3
E) CH3 - SH
A)

B)
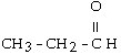
C) CH3 - CH= CH - CH₂ - CH3
D) CH3 - CH₂ - O - CH₂ - CH3
E) CH3 - SH

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
7
The common name for 2-butanone, a readily available solvent, is
A) methyl acetone.
B) methyl ethyl ketone or MEK.
C) β-butanone.
D) butyl ketone.
E) butyl ether.
A) methyl acetone.
B) methyl ethyl ketone or MEK.
C) β-butanone.
D) butyl ketone.
E) butyl ether.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
8
How many carbonyl-containing isomers does the formula C₃H6O have?
A) two
B) three
C) five
D) seven
E) eight
A) two
B) three
C) five
D) seven
E) eight

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
9
What is the IUPAC name for this compound? 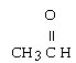
A) methyl aldehyde
B) 1-ethanaldehyde
C) 1-ethanone
D) ethanal
E) methanal
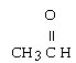
A) methyl aldehyde
B) 1-ethanaldehyde
C) 1-ethanone
D) ethanal
E) methanal

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
10
The carbonyl group consists of
A) a carbon-oxygen-hydrogen structure.
B) a carbon-oxygen single bond.
C) a carbon-oxygen double bond.
D) a carbon-oxygen triple bond.
E) a carbon-oxygen-carbon structure.
A) a carbon-oxygen-hydrogen structure.
B) a carbon-oxygen single bond.
C) a carbon-oxygen double bond.
D) a carbon-oxygen triple bond.
E) a carbon-oxygen-carbon structure.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
11
The oxygen atom in a carbonyl group is __________ the carbon atom.
A) more electronegative than
B) less electronegative than
C) identical in electronegativity to
D) more electropositive than
E) more soluble than
A) more electronegative than
B) less electronegative than
C) identical in electronegativity to
D) more electropositive than
E) more soluble than

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
12
In the IUPAC naming system, an aldehyde is named by replacing the -e of the name of the corresponding alkane with
A) yne.
B) ene.
C) al.
D) one.
E) ol.
A) yne.
B) ene.
C) al.
D) one.
E) ol.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
13
In all aldehydes except formaldehyde, how many hydrogen atoms is the carbonyl group bonded to?
A) one
B) two
C) three
D) four
A) one
B) two
C) three
D) four

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
14
In the IUPAC naming system, a ketone is named by replacing the -e in the corresponding alkane name with
A) yne.
B) ene.
C) al.
D) one.
E) ol.
A) yne.
B) ene.
C) al.
D) one.
E) ol.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
15
What is the name of this compound? 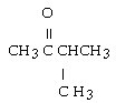
A) 2-pentanone
B) methyl propyl ketone
C) 3-methyl-2-butanone
D) 2-methyl-3-butanone
E) 2-methyl-3-ketone butane

A) 2-pentanone
B) methyl propyl ketone
C) 3-methyl-2-butanone
D) 2-methyl-3-butanone
E) 2-methyl-3-ketone butane

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
16
The compound 2-propanone is also known as
A) acetone.
B) 2-propanone.
C) dimethyl ketone.
D) β-propanone.
E) propylone.
A) acetone.
B) 2-propanone.
C) dimethyl ketone.
D) β-propanone.
E) propylone.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
17
Which of the following pairs of compounds are isomers?
A) CH3CH₂CH₂OH and CH3O CH3
B)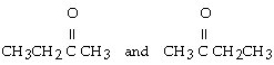
C)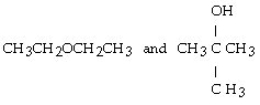
D)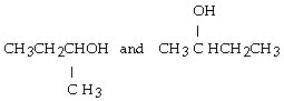
E)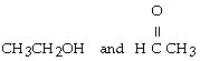
A) CH3CH₂CH₂OH and CH3O CH3
B)
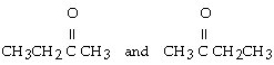
C)
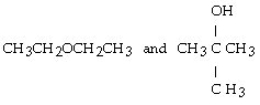
D)
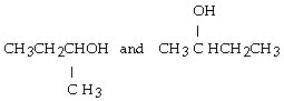
E)
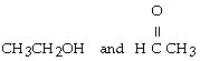

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
18
Which of the following compounds contains a ketone functional group?
A)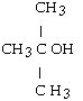
B)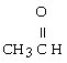
C) CH3CH₂OCH₂CH3
D)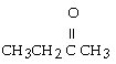
E)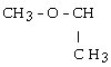
A)

B)
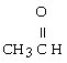
C) CH3CH₂OCH₂CH3
D)
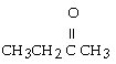
E)
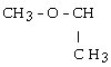

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
19
Three functional groups found in this compound are 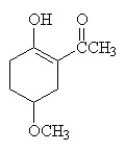
A) alcohol, aromatic, and ether.
B) alcohol, aldehyde, and ether.
C) alcohol, ether, and ketone.
D) aldehyde, ether, and carboxylic acid.
E) cycloalkene, alcohol, and carboxylic acid.

A) alcohol, aromatic, and ether.
B) alcohol, aldehyde, and ether.
C) alcohol, ether, and ketone.
D) aldehyde, ether, and carboxylic acid.
E) cycloalkene, alcohol, and carboxylic acid.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
20
Acetone can be produced by the body when a person is
A) exercising.
B) dieting with high protein diets.
C) ill with a flu.
D) recovering from surgery.
E) sleeping.
A) exercising.
B) dieting with high protein diets.
C) ill with a flu.
D) recovering from surgery.
E) sleeping.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
21
Which of the compounds would give a positive Tollens test?
A)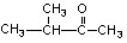
B)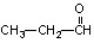
C)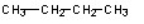
D)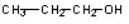
E)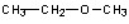
A)
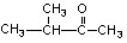
B)
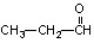
C)
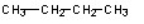
D)
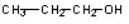
E)
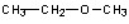

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
22
The Tollens test may be used to distinguish
A) acids from amines.
B) esters from acids.
C) ketones from alcohols.
D) aldehydes from ketones.
E) alcohols from alkenes.
A) acids from amines.
B) esters from acids.
C) ketones from alcohols.
D) aldehydes from ketones.
E) alcohols from alkenes.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
23
Aldehydes and ketones may be reduced to
A) acids.
B) alkanes.
C) ethers.
D) alcohols.
E) esters.
A) acids.
B) alkanes.
C) ethers.
D) alcohols.
E) esters.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
24
An acetal is formed from two molecules of an alcohol and a(n)
A) aldehyde.
B) ether.
C) carboxylic acid.
D) alkyl ether.
E) ester.
A) aldehyde.
B) ether.
C) carboxylic acid.
D) alkyl ether.
E) ester.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
25
The hydrogenation of 2-methylpropanal gives the product
A) 1-butanol.
B) 2-methylpropanoic acid.
C) 2-methyl-3-propanol.
D) 2-methyl-1-propanol.
E) 2-methyl-2-propanol.
A) 1-butanol.
B) 2-methylpropanoic acid.
C) 2-methyl-3-propanol.
D) 2-methyl-1-propanol.
E) 2-methyl-2-propanol.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
26
The ketone with the constitutional formula C5H10O can also be written as
A) CH3CH₂CH₂COCH3.
B) CH3COCH₂CH₂CH3.
C) CH3CH₂COCH₂CH3.
D) All of the above.
E) None of the above.
A) CH3CH₂CH₂COCH3.
B) CH3COCH₂CH₂CH3.
C) CH3CH₂COCH₂CH3.
D) All of the above.
E) None of the above.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
27
How many moles of an alcohol are needed to react with 1 mole of an aldehyde to form a hemiacetal?
A) 1
B) 1.5
C) 2
D) 3
E) 3.5
A) 1
B) 1.5
C) 2
D) 3
E) 3.5

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
28
The reduction of 3-pentanone with hydrogen in the presence of a nickel catalyst will yield
A) pentane.
B) 2-pentene.
C) diethyl alcohol.
D) 3-pentanol.
E) pentanaldehyde.
A) pentane.
B) 2-pentene.
C) diethyl alcohol.
D) 3-pentanol.
E) pentanaldehyde.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
29
Low-molecular-weight ketones are soluble in water. What is the shortest length of the carbon chain where insolubility becomes important?
A) one
B) two
C) four
D) five
E) eight
A) one
B) two
C) four
D) five
E) eight

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
30
The flavoring agent found in vanilla is
A) an aldehyde.
B) a ketone.
C) a hydrocarbon.
D) an ester.
E) a thiol.
A) an aldehyde.
B) a ketone.
C) a hydrocarbon.
D) an ester.
E) a thiol.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
31
The increased boiling point of ketones compared to alkanes and ethers of similar mass is due to
A) hydrogen bonding.
B) dipole-dipole interactions.
C) a bent chain structure.
D) resonance.
E) ionic interactions.
A) hydrogen bonding.
B) dipole-dipole interactions.
C) a bent chain structure.
D) resonance.
E) ionic interactions.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
32
What is the name of this compound? 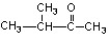
A) 2-methylbutanone
B) 3-methyl-2-butanone
C) 2-methyl-3-butanone
D) 3-methylbutanone
E) pentanone
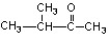
A) 2-methylbutanone
B) 3-methyl-2-butanone
C) 2-methyl-3-butanone
D) 3-methylbutanone
E) pentanone

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
33
The addition of hydrogen to an organic compound or the loss of oxygen is called
A) reduction.
B) oxidation.
C) dehydration.
D) halogenation.
E) hydration.
A) reduction.
B) oxidation.
C) dehydration.
D) halogenation.
E) hydration.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
34
Which of the following would not be water soluble?
A) acetone
B) propanal
C) 3-heptanone
D) formaldehyde
E) 2-butanone
A) acetone
B) propanal
C) 3-heptanone
D) formaldehyde
E) 2-butanone

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
35
Benedict's test requires an aldehyde and an adjacent
A) saturated carbon.
B) ketone.
C) alcohol.
D) phenyl ring.
E) acid.
A) saturated carbon.
B) ketone.
C) alcohol.
D) phenyl ring.
E) acid.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
36
Formalin is
A) another name for formaldehyde.
B) a plastic.
C) an aqueous solution of formaldehyde.
D) an excellent solvent.
E) a polymer.
A) another name for formaldehyde.
B) a plastic.
C) an aqueous solution of formaldehyde.
D) an excellent solvent.
E) a polymer.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
37
Aldehydes have higher boiling points than alkanes of similar mass because of
A) hydrogen bonding.
B) dipole-dipole interactions.
C) ionic bonding.
D) covalent bonding.
E) oxygen bonding.
A) hydrogen bonding.
B) dipole-dipole interactions.
C) ionic bonding.
D) covalent bonding.
E) oxygen bonding.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
38
Acetone is a ketone commonly used as a
A) preservative.
B) flavoring agent.
C) fuel.
D) solvent.
E) drain cleaner.
A) preservative.
B) flavoring agent.
C) fuel.
D) solvent.
E) drain cleaner.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
39
Which of the following ketones is the most soluble in water?
A)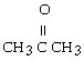
B)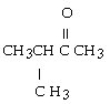
C)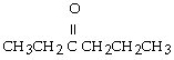
D)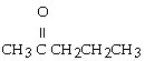
E)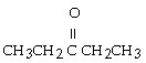
A)
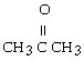
B)

C)
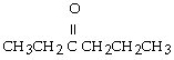
D)
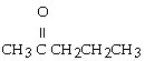
E)


Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
40
How do sugars form cyclic hemiacetals?
A) Two molecules of a sugar react with one another.
B) A molecule of sugar reacts with an added alcohol.
C) A molecule of sugar reacts with itself.
D) A molecule of sugar reacts with an added aldehyde.
E) A sugar molecule decomposes.
A) Two molecules of a sugar react with one another.
B) A molecule of sugar reacts with an added alcohol.
C) A molecule of sugar reacts with itself.
D) A molecule of sugar reacts with an added aldehyde.
E) A sugar molecule decomposes.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
41
The carbonyl group consists of a carbon-oxygen single bond, and a second bond to hydrogen.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
42
The product of adding two molecules of an alcohol to an aldehyde in the presence of acid is a(n)
A) acetal.
B) ether.
C) hemiacetal.
D) hemiether.
E) hydroxyl group.
A) acetal.
B) ether.
C) hemiacetal.
D) hemiether.
E) hydroxyl group.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
43
An enantiomer is
A) a stereoisomer that is not a mirror image of another molecule.
B) a stereoisomer that is a mirror image of another molecule.
C) a diastereoisomer.
D) a structural isomer.
E) a cis-trans isomer.
A) a stereoisomer that is not a mirror image of another molecule.
B) a stereoisomer that is a mirror image of another molecule.
C) a diastereoisomer.
D) a structural isomer.
E) a cis-trans isomer.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
44
The suffix -al indicates an aldehyde in the IUPAC system of naming.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
45
Chirality occurs when stereoisomers have mirror images that are
A) superimposable.
B) the same.
C) not superimposable.
D) not visible to one another.
E) identical.
A) superimposable.
B) the same.
C) not superimposable.
D) not visible to one another.
E) identical.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
46
Many odors from solvents, paint removers, and perfumes are derived from aldehydes or ketones.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
47
Achiral compounds are those which
A) have no "handedness."
B) have different mirror images.
C) are non-superimposable.
D) have the same formula but different structures.
E) are a racemic mixture.
A) have no "handedness."
B) have different mirror images.
C) are non-superimposable.
D) have the same formula but different structures.
E) are a racemic mixture.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
48
Which of these compounds is the hemiacetal that forms when ethanol reacts with propanal?
A)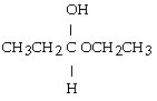
B)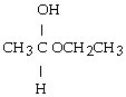
C)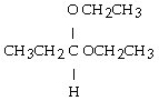
D)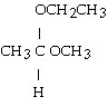
E)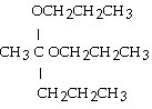
A)
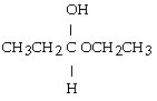
B)
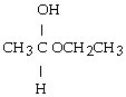
C)
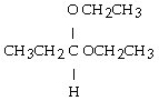
D)
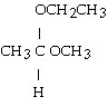
E)
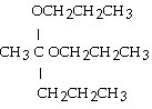

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
49
What is the acetal formed when propanone reacts with two molecules of methanol?
A)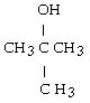
B)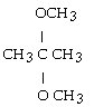
C)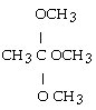
D)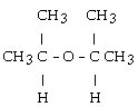
E)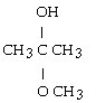
A)

B)

C)
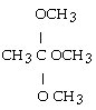
D)

E)


Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
50
Which molecule below has stereoisomers with different biological effects?
A) carvone
B) nicotine
C) LSD
D) epinephrine
E) All of the above.
A) carvone
B) nicotine
C) LSD
D) epinephrine
E) All of the above.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
51
Acetone is a three-carbon aldehyde.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
52
In a ketone, the carbonyl group is bonded to two other carbon atoms.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
53
Acetone is sometimes produced in pathological conditions such as diabetes.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
54
How many different substituents are required on a carbon atom for it to be chiral?
A) one
B) two
C) three
D) four
E) Any number from 1 to 4; chiralty does not depend on substitution.
A) one
B) two
C) three
D) four
E) Any number from 1 to 4; chiralty does not depend on substitution.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
55
The carbonyl group does not have a dipole.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
56
The suffix -one indicates an aldehyde in the IUPAC system of naming.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
57
All aldehydes have a carbonyl carbon bonded to at least two hydrogens.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
58
Formaldehyde is used in solution as a germicide and preservative.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
59
Excessive exposure to formaldehyde can irritate the eyes and respiratory tract.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
60
Chiral drugs consist of only one enantiomer. The benefits of using a pure enantiomer, rather than a mixture, include
A) higher potency (lower total dose of drug).
B) elimination of side effects.
C) reduced chances of drug interactions.
D) All of the above.
E) None of the above.
A) higher potency (lower total dose of drug).
B) elimination of side effects.
C) reduced chances of drug interactions.
D) All of the above.
E) None of the above.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
61
Propanal is more soluble than pentanal.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
62
Benedict's test can be used to determine whether an α-hydroxyaldehyde functional group is present.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
63
An acetal is formed from an aldehyde or ketone and one molecule of alcohol.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
64
The carbonyl group gives aldehydes higher boiling points than alkanes of similar mass.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
65
Primary alcohols are oxidized to ketones.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
66
A hemiacetal has alkoxy and hydroxyl functional groups bonded to the same carbon.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
67
A chiral carbon atom can have fewer than four different groups bonded to it.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
68
Ketones are reduced to secondary alcohols.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
69
The carbonyl group gives ketones lower boiling points than alkanes of similar mass.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
70
Acetone would give a positive Tollens test.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
71
1-butanol is a chiral molecule.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
72
The Tollens test is used to distinguish between aldehydes and acids.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
73
Hexanal would be soluble in water.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
74
A major flavor component of vanilla is an aldehyde.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
75
Glucose forms a cyclic hemiacetal.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
76
Enantiomers may have very different tastes or smells.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
77
Enantiomers are mirror images of each other.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
78
Carbonyl compounds having fewer than four carbon atoms are very water soluble.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
79
Enantiomers have superimposable mirror images.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
80
2-butanol is a chiral molecule.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck



