Deck 12: Alkenes, Alkynes, and Aromatic Compounds
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/81
Play
Full screen (f)
Deck 12: Alkenes, Alkynes, and Aromatic Compounds
1
The compound 1-butyne contains
A) all single bonds.
B) a double bond.
C) a triple bond.
D) a ring structure.
E) a bromine atom.
A) all single bonds.
B) a double bond.
C) a triple bond.
D) a ring structure.
E) a bromine atom.
a triple bond.
2
Which of the compounds is a cycloalkene?
A) CH₂ = CHCH = CH₂
B)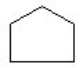
C) CH3C = CH₂
D)
E)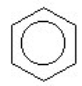
A) CH₂ = CHCH = CH₂
B)

C) CH3C = CH₂
D)

E)


3
What is the condensed structural formula of the compound propene?
A) CH3CH₂CH3
B) H3C = CH₂CH3
C) H₂C = C = CH₂
D) CH3CH = CH₂
E) HC ≡ CCH3
A) CH3CH₂CH3
B) H3C = CH₂CH3
C) H₂C = C = CH₂
D) CH3CH = CH₂
E) HC ≡ CCH3
CH3CH = CH₂
4
What is the IUPAC name for the following compound? 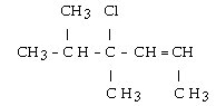
A) 4-chloro-4,5-dimethyl-2-hexene
B) 3-chloro-1,3,4-trimethyl-1-pentene
C) 3-chloro-2,3-dimethyl-4-hexene
D) 3-chloro-2,3,5-trimethyl-4-pentene
E) 3-chloro-1,3,4,4-tetramethyl-1-butene
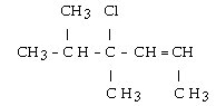
A) 4-chloro-4,5-dimethyl-2-hexene
B) 3-chloro-1,3,4-trimethyl-1-pentene
C) 3-chloro-2,3-dimethyl-4-hexene
D) 3-chloro-2,3,5-trimethyl-4-pentene
E) 3-chloro-1,3,4,4-tetramethyl-1-butene

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
5
Which of the following compounds is an alkyne?
A) CH3CH₂CH3
B) C₃H6
C) CH3CH₂C ≡ CH
D) H₂C = CHCH = CH₂
E) 2-pentene
A) CH3CH₂CH3
B) C₃H6
C) CH3CH₂C ≡ CH
D) H₂C = CHCH = CH₂
E) 2-pentene

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
6
A hydrocarbon with a double bond is a(n)
A) alkane.
B) alkene.
C) alkyne.
D) alcohol.
E) saturated compound.
A) alkane.
B) alkene.
C) alkyne.
D) alcohol.
E) saturated compound.

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
7
The IUPAC name for ethylene is
A) ethane.
B) cycloethane.
C) ethyne.
D) ethanene.
E) ethene.
A) ethane.
B) cycloethane.
C) ethyne.
D) ethanene.
E) ethene.

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
8
Name the following compound. 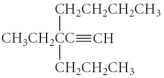
A) 3-butyl-3-propyl-1-pentyne
B) 3-butyl-3-propyl-4-pentyne
C) 3-ethyl-3-propyl-1-heptyne
D) 5-ethyl-5-propyl-6-heptyne
E) 3-ethyl-3-butyl-1-hexyne
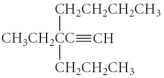
A) 3-butyl-3-propyl-1-pentyne
B) 3-butyl-3-propyl-4-pentyne
C) 3-ethyl-3-propyl-1-heptyne
D) 5-ethyl-5-propyl-6-heptyne
E) 3-ethyl-3-butyl-1-hexyne

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
9
The IUPAC name for CH3CH₂C ≡ CCH3 is
A) 3-pentyne.
B) 2-pentyne.
C) pentyne.
D) 1-methylbutyne.
E) 2-propene.
A) 3-pentyne.
B) 2-pentyne.
C) pentyne.
D) 1-methylbutyne.
E) 2-propene.

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
10
Name the following compound. 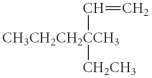
A) 2,2-diethylpenatane
B) 2,2-diethylpentene
C) 4-ethyl-4-methyl-5-hexene
D) 3-ethyl-3-methyl-1-hexene
E) 4-ethyl-4-methylhexane
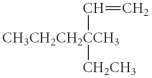
A) 2,2-diethylpenatane
B) 2,2-diethylpentene
C) 4-ethyl-4-methyl-5-hexene
D) 3-ethyl-3-methyl-1-hexene
E) 4-ethyl-4-methylhexane

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
11
Which one of the following compounds has the smallest number of hydrogen atoms?
A) butyne
B) 2-methylpropane
C) butene
D) 2-methylcyclopropane
E) butane
A) butyne
B) 2-methylpropane
C) butene
D) 2-methylcyclopropane
E) butane

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
12
When naming an alkene, the parent chain is the longest carbon chain
A) that does not contain the double bond.
B) regardless of whether or not it contains the double bond.
C) that contains at least one of the carbon atoms of the double bond.
D) that contains both atoms of the double bond.
E) that contains a branch.
A) that does not contain the double bond.
B) regardless of whether or not it contains the double bond.
C) that contains at least one of the carbon atoms of the double bond.
D) that contains both atoms of the double bond.
E) that contains a branch.

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
13
Alkenes and alkynes are called unsaturated compounds because
A) they have the maximum number of hydrogen atoms attached to each carbon in the compound.
B) they have fewer hydrogen atoms attached to the carbon chain than alkanes.
C) they have more hydrogen atoms attached to the carbon chain than alkanes.
D) they have more carbon atoms than alkanes.
E) they have fewer carbon atoms than alkanes.
A) they have the maximum number of hydrogen atoms attached to each carbon in the compound.
B) they have fewer hydrogen atoms attached to the carbon chain than alkanes.
C) they have more hydrogen atoms attached to the carbon chain than alkanes.
D) they have more carbon atoms than alkanes.
E) they have fewer carbon atoms than alkanes.

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
14
The carbon atoms in saturated hydrocarbons
A) have only single bonds.
B) contain at least one double bond.
C) contain at least one triple bond.
D) contain a benzene ring.
E) contain both a double and a triple bond.
A) have only single bonds.
B) contain at least one double bond.
C) contain at least one triple bond.
D) contain a benzene ring.
E) contain both a double and a triple bond.

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
15
Organic compounds with double or triple bonds are classified as
A) unsaturated compounds.
B) saturated compounds.
C) dilute solutions.
D) concentrated solutions.
E) substituted compounds.
A) unsaturated compounds.
B) saturated compounds.
C) dilute solutions.
D) concentrated solutions.
E) substituted compounds.

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
16
Which of the following is the IUPAC name for the compound below? 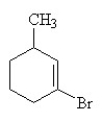
A) m-bromotoluene
B) m-bromomethylcyclohexene
C) 1-bromo-3-methylcyclohexene
D) 3-bromo-1-methyl-2-cyclohexene
E) 2-bromo-6-methylcyclohexene

A) m-bromotoluene
B) m-bromomethylcyclohexene
C) 1-bromo-3-methylcyclohexene
D) 3-bromo-1-methyl-2-cyclohexene
E) 2-bromo-6-methylcyclohexene

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
17
What is the condensed structural formula for the compound 3-hexene?
A)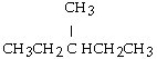
B) CH₂= CHCH₂CH₂CH₂CH3
C) CH3CH₂CH = CHCH₂CH3
D) CH3CH₂CH₂CH = CHCH3
E) CH3CH = CHCH₂CH₂CH3
A)
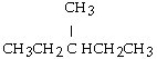
B) CH₂= CHCH₂CH₂CH₂CH3
C) CH3CH₂CH = CHCH₂CH3
D) CH3CH₂CH₂CH = CHCH3
E) CH3CH = CHCH₂CH₂CH3

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
18
Which of the following names are correct?
A) 3-butyl-4-pentene
B) 3-ethyl-1-heptene
C) 3-etheneheptane
D) 5-ethyl-6-heptene
E) All of the above are correct.
A) 3-butyl-4-pentene
B) 3-ethyl-1-heptene
C) 3-etheneheptane
D) 5-ethyl-6-heptene
E) All of the above are correct.

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
19
The IUPAC name of CH3CH = CHCH3 is
A) 2-butene.
B) 2-butane.
C) 1-butene.
D) butene.
E) 2-butyne.
A) 2-butene.
B) 2-butane.
C) 1-butene.
D) butene.
E) 2-butyne.

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
20
An unsaturated compound always
A) is a cycloalkane.
B) contains a double bond.
C) contains a triple bond.
D) contains at least one double or triple bond.
E) is aromatic.
A) is a cycloalkane.
B) contains a double bond.
C) contains a triple bond.
D) contains at least one double or triple bond.
E) is aromatic.

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
21
Which of the following pairs of compounds are cis-trans isomers?
A)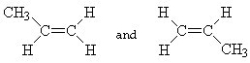
B)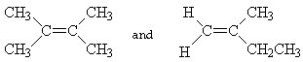
C) HC ≡ C - CH3 and CH3- C ≡ CH
D)
E)
A)
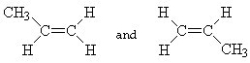
B)
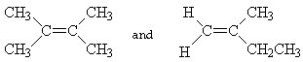
C) HC ≡ C - CH3 and CH3- C ≡ CH
D)

E)


Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
22
The chemical reaction of 2-butene and HCl yields what product?
A) CH3CH₂CH₂CH3
B)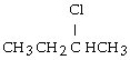
C)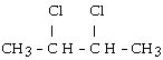
D)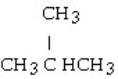
E)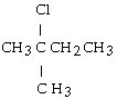
A) CH3CH₂CH₂CH3
B)
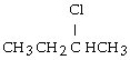
C)
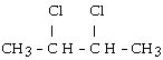
D)
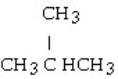
E)


Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
23
According to Markovnikov's rule, the hydrogen in HCl adds to the carbon in the double bond
A) attached to the end carbon.
B) that has the smaller number of hydrogen atoms attached.
C) that has the greater number of hydrogen atoms attached.
D) that has the smaller number of carbon atoms attached.
E) that has the greater number of carbon atoms attached.
A) attached to the end carbon.
B) that has the smaller number of hydrogen atoms attached.
C) that has the greater number of hydrogen atoms attached.
D) that has the smaller number of carbon atoms attached.
E) that has the greater number of carbon atoms attached.

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
24
The reaction of hydrogen (H₂) and propene using a platinum catalyst is called
A) combustion.
B) substitution.
C) addition.
D) neutralization.
E) condensation.
A) combustion.
B) substitution.
C) addition.
D) neutralization.
E) condensation.

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
25
Which of the following is the correct structural formula for 3-methylcyclohexene?
A)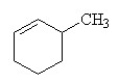
B)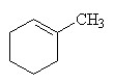
C)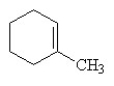
D)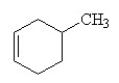
E)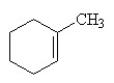
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
26
Insects communicate with chemicals called
A) markers.
B) isomers.
C) signals.
D) pheromones.
E) scents.
A) markers.
B) isomers.
C) signals.
D) pheromones.
E) scents.

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
27
The reaction of an alkene and water in the presence of an acid catalyst to produce an alcohol is called
A) hydrolysis.
B) alkoholysis.
C) halogenation.
D) hydration.
E) hydrohydration.
A) hydrolysis.
B) alkoholysis.
C) halogenation.
D) hydration.
E) hydrohydration.

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
28
What is the name of the compound shown below? 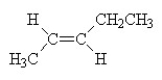
A) 2-pentene
B) trans-2-pentene
C) trans-3-pentene
D) cis-2-pentene
E) cis-3-pentene

A) 2-pentene
B) trans-2-pentene
C) trans-3-pentene
D) cis-2-pentene
E) cis-3-pentene

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
29
The hydrogenation of an alkene gives a(n).
A) alkane.
B) alkene.
C) alkyne.
D) benzene.
E) isomer.
A) alkane.
B) alkene.
C) alkyne.
D) benzene.
E) isomer.

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
30
Some alkenes have cis-trans isomers because
A) the carbon atoms in the double bond cannot rotate.
B) each of the carbon atoms in the double bond has four different groups attached to it.
C) one of the carbon atoms in the double bond has two identical groups attached to it.
D) the carbon atoms in the double bond are free to rotate.
E) all of the carbon atoms in the compound are rigid and cannot rotate.
A) the carbon atoms in the double bond cannot rotate.
B) each of the carbon atoms in the double bond has four different groups attached to it.
C) one of the carbon atoms in the double bond has two identical groups attached to it.
D) the carbon atoms in the double bond are free to rotate.
E) all of the carbon atoms in the compound are rigid and cannot rotate.

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
31
The reactants needed to produce the compound chlorocyclopentane are
A) pentene and HCl.
B) pentene and Cl2
C) cyclopentane and Cl2
D) cyclopentene and HCl.
E) cyclopentene and Cl2
A) pentene and HCl.
B) pentene and Cl2
C) cyclopentane and Cl2
D) cyclopentene and HCl.
E) cyclopentene and Cl2

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
32
The major product of the reaction of 3-methyl-2-pentene with HCl is
A) 4-chloro-3-methylpentane.
B) 1-chloro-3-methylpentane.
C) 2-chloro-3-methylpentane.
D) 3-chloro-3-methylpentane.
E) 3-chloro-3-methylpentene.
A) 4-chloro-3-methylpentane.
B) 1-chloro-3-methylpentane.
C) 2-chloro-3-methylpentane.
D) 3-chloro-3-methylpentane.
E) 3-chloro-3-methylpentene.

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
33
Which of the following compounds exhibit geometric isomerism?
A) CH₂=CH-CH3
B) CCl₂=CBr₂
C) CH3-CH=CH-CH3
D) CCl₂=CHBr
E) All of the above exhibit geometric isomerism.
A) CH₂=CH-CH3
B) CCl₂=CBr₂
C) CH3-CH=CH-CH3
D) CCl₂=CHBr
E) All of the above exhibit geometric isomerism.

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
34
The reaction of propene and Br₂ yields
A) 1-bromopropane.
B) 2-bromopropane.
C) 1,1-dibromopropane.
D) 1,2-dibromopropane.
E) 2,2-dibromopropane.
A) 1-bromopropane.
B) 2-bromopropane.
C) 1,1-dibromopropane.
D) 1,2-dibromopropane.
E) 2,2-dibromopropane.

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
35
What is the condensed structural formula for the product of the hydrogenation of 2-butene using a platinum catalyst?
A) CH3CH = CHCH3
B) Cl |
CH3CH₂CHCH3
C) CH3CH₂CH₂CH3
D)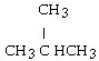
E)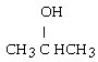
A) CH3CH = CHCH3
B) Cl |
CH3CH₂CHCH3
C) CH3CH₂CH₂CH3
D)
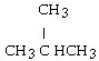
E)
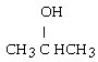

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
36
What is the major product of the reaction shown below? CH3- CH₂- CH = CH₂ + HOH 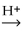
A)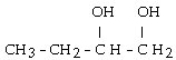
B)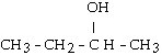
C) CH3-CH₂-CH₂-CH₂- OH
D)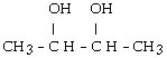
E)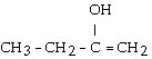
A)
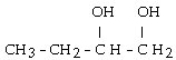
B)
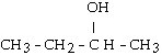
C) CH3-CH₂-CH₂-CH₂- OH
D)
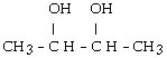
E)
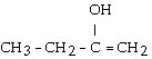

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
37
What is the product of this reaction? CH3CH₂CH = CHCH3 + Cl₂ → ?
A) CH3CH₂CH₂CH₂CH3
B)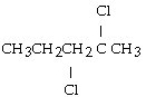
C)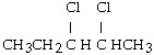
D)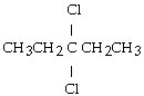
E)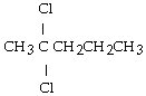
A) CH3CH₂CH₂CH₂CH3
B)
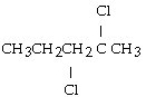
C)
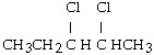
D)

E)
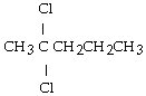

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
38
The reaction of cyclohexene and Cl₂ yields
A) 1-chlorocyclohexene.
B) 1,2-dichlorocyclohexane.
C) 1,3-dichlorocyclohexane.
D) 2,3-dichlorocyclohexane.
E) 3,4-dichlorocyclohexane.
A) 1-chlorocyclohexene.
B) 1,2-dichlorocyclohexane.
C) 1,3-dichlorocyclohexane.
D) 2,3-dichlorocyclohexane.
E) 3,4-dichlorocyclohexane.

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
39
The odors you associate with lemons, oranges, roses, and lavender are due to
A) alkenes.
B) alkanes.
C) alkynes.
D) thiols.
E) amines.
A) alkenes.
B) alkanes.
C) alkynes.
D) thiols.
E) amines.

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
40
Which set of reactants would give this compound? 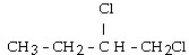
A) CH3CH?CH?CH3 + 2HCl
B) CH3CH CHCH3 + 2HCl
C) CH3CH?CH CH? + 2HCl
D) CH3CH CHCH3 + Cl?
E) CH3CH?CH CH? + Cl?
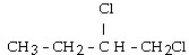
A) CH3CH?CH?CH3 + 2HCl
B) CH3CH CHCH3 + 2HCl
C) CH3CH?CH CH? + 2HCl
D) CH3CH CHCH3 + Cl?
E) CH3CH?CH CH? + Cl?

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
41
A compound that contains the ring structure of benzene is called a(n)
A) alkane.
B) cycloalkane.
C) alkyl group.
D) aromatic compound.
E) hydrocarbon.
A) alkane.
B) cycloalkane.
C) alkyl group.
D) aromatic compound.
E) hydrocarbon.

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
42
The compound below is named 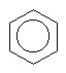
A) cyclohexane.
B) cyclohexene.
C) cyclohexyne.
D) benzene.
E) cyclobenzene.

A) cyclohexane.
B) cyclohexene.
C) cyclohexyne.
D) benzene.
E) cyclobenzene.

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
43
How many isomers are there for dibromobenzene?
A) one
B) two
C) three
D) four
E) five
A) one
B) two
C) three
D) four
E) five

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
44
Small molecules that make up the repeat unit in polymers are called
A) monomers.
B) alkenes.
C) alkynes.
D) minipolymers.
E) synthetic polymers.
A) monomers.
B) alkenes.
C) alkynes.
D) minipolymers.
E) synthetic polymers.

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
45
Alkynes contain double bonds.

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
46
The name of the compound shown below is 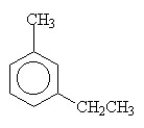
A) o-ethylmethylcyclohexane.
B) m-ethylmethylcyclohexane.
C) o-ethyltoluene.
D) m-ethyltoluene.
E) p-ethyltoluene.

A) o-ethylmethylcyclohexane.
B) m-ethylmethylcyclohexane.
C) o-ethyltoluene.
D) m-ethyltoluene.
E) p-ethyltoluene.

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
47
The structural formula of benzene is often represented as a
A) ring of five carbon atoms.
B) ring of six carbon atoms with six double bonds.
C) ring of six carbon atoms with a circle in the center.
D) cycloalkane.
E) cycloalkyne.
A) ring of five carbon atoms.
B) ring of six carbon atoms with six double bonds.
C) ring of six carbon atoms with a circle in the center.
D) cycloalkane.
E) cycloalkyne.

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
48
Which of the following is a naturally occurring polymer (not a synthetic polymer)?
A) polypropylene
B) DNA
C) teflon
D) polystyrene
E) saran
A) polypropylene
B) DNA
C) teflon
D) polystyrene
E) saran

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
49
All of the carbon-carbon bonds in benzene are
A) composed of only two types, single and double.
B) identical.
C) double bonds.
D) single bonds.
E) circular bonds.
A) composed of only two types, single and double.
B) identical.
C) double bonds.
D) single bonds.
E) circular bonds.

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
50
What are the prefixes used to designate substituent positions on disubstituted benzene compounds?
A) ortho-, meta-, and para-.
B) cis- and trans-
C) syn- and anti-
D) All of the above.
E) None of the above.
A) ortho-, meta-, and para-.
B) cis- and trans-
C) syn- and anti-
D) All of the above.
E) None of the above.

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
51
Which of the following names are correct?
A) 4-bromo-2-ethylbenzene
B) 1-chloro-3-propylbenzene
C) 3-bromo-4-chlorobenzene
D) 2,2-dibromobenzene
E) 4-fluoro-2-iodobenzene
A) 4-bromo-2-ethylbenzene
B) 1-chloro-3-propylbenzene
C) 3-bromo-4-chlorobenzene
D) 2,2-dibromobenzene
E) 4-fluoro-2-iodobenzene

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
52
Propylene is used to induce ripening in fruits.

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
53
What is the starting monomer for the polymer Teflon? 
A)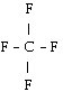
B)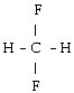
C)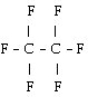
D) C F
E)

A)
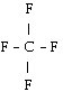
B)

C)

D) C F
E)

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
54
When chlorine atoms are attached to carbon 1 and carbon 3 in benzene, the compound is named
A) dichlorobenzene.
B) o-dichlorobenzene.
C) m-dichlorobenzene.
D) p-dichlorobenzene.
E) j-dichlorobenzene.
A) dichlorobenzene.
B) o-dichlorobenzene.
C) m-dichlorobenzene.
D) p-dichlorobenzene.
E) j-dichlorobenzene.

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
55
The synthetic polymer polyethylene is unreactive because it is
A) unsaturated.
B) a haloalkane.
C) saturated.
D) a cycloalkene.
E) an aromatic compound.
A) unsaturated.
B) a haloalkane.
C) saturated.
D) a cycloalkene.
E) an aromatic compound.

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
56
What is the molecular formula of benzene?
A) C6H4
B) C6H6
C) C6H₈
D) C6H10
E) C6H12
A) C6H4
B) C6H6
C) C6H₈
D) C6H10
E) C6H12

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
57
When bromine atoms are attached to carbon 1 and carbon 4 in benzene, the compound is named
A) dibromobenzene.
B) o-dibromobenzene.
C) m-dibromobenzene.
D) p-dibromobenzene.
E) f-dibromobenzene.
A) dibromobenzene.
B) o-dibromobenzene.
C) m-dibromobenzene.
D) p-dibromobenzene.
E) f-dibromobenzene.

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
58
What is the name of the compound below? 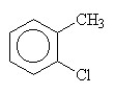
A) p-chlorotoluene
B) o-chlorotoluene
C) m-chlorotoluene
D) 1-chloro-2-methyltoluene
E) 2-chloro-1-methyltoluene

A) p-chlorotoluene
B) o-chlorotoluene
C) m-chlorotoluene
D) 1-chloro-2-methyltoluene
E) 2-chloro-1-methyltoluene

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
59
Which of the following would result from the polymerization of ethene?
A)
B)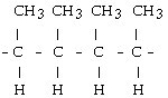
C)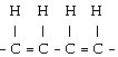
D) = C = C = C = C =
E) - CH₂- CH₂- CH₂- CH₂-
A)

B)
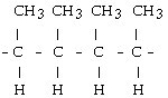
C)
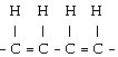
D) = C = C = C = C =
E) - CH₂- CH₂- CH₂- CH₂-

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
60
Long-chain molecules that consist of many repeating units are called
A) polymers.
B) monomers.
C) organic compounds.
D) alkenes.
E) alkanes.
A) polymers.
B) monomers.
C) organic compounds.
D) alkenes.
E) alkanes.

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
61
In a cis alkene, the groups are on the same side of the double bond.

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
62
The substituent group C6H5CH₂- is called a phenyl group.

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
63
Ortho compounds have substituents on the 1 and 3 positions of the benzene ring.

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
64
Water can be added to alkenes to produce acids.

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
65
Markovnikov's rule states that when a hydrogen halide adds to a double bond, the proton will bond to the carbon that has the greater number of hydrogen atoms.

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
66
Fragrances and flavors are often compounds with more than one functional group (for example, an alkene that also contains an aldehyde).

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
67
Polymers are large molecules consisting of repeating units.

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
68
One essential building block of aspirin, ibuprofen, and acetaminophen is the benzene ring.

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
69
Halogenation is the process of adding chlorine or similar elements to an alkene or alkyne.

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
70
Para compounds have substituents on the 1 and 4 positions of the benzene ring.

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
71
Light-induced cis-trans isomerization is an important step in vision.

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
72
Nylon, polyester, and most other plastics are carbon compounds.

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
73
Hydrogenation of unsaturated vegetable oils raises the melting point and makes them more solid.

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
74
In a trans alkene, the groups are on the same side of the double bond.

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
75
Hydration is used to convert alkenes and alkynes to alkanes.

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
76
Most products made from polymers can be recycled.

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
77
Alkynes can show cis-trans isomerism.

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
78
An alkynes containing three carbons is named propyne.

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
79
Hydrogenation is used to convert alkenes and alkynes to alkanes.

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
80
All alkenes show cis-trans isomerism.

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck



