Deck 12: Chemical Bonding
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/45
Play
Full screen (f)
Deck 12: Chemical Bonding
1
Which of the following is another term for unshared electron pairs?
A)Covalent pairs
B)Ionic pairs
C)Valence pairs
D)Lone pairs
E)Bonding electron pairs
A)Covalent pairs
B)Ionic pairs
C)Valence pairs
D)Lone pairs
E)Bonding electron pairs
Lone pairs
2
Which of these are both isoelectronic with noble gas atoms?
A)Fe2+ and S2-
B)Sc3+ and Cl-
C)Ca2+ and H+
D)Ag+ and P3-
E)Fe3+ and Fe2+
A)Fe2+ and S2-
B)Sc3+ and Cl-
C)Ca2+ and H+
D)Ag+ and P3-
E)Fe3+ and Fe2+
Sc3+ and Cl-
3
In a covalent bond,the valence electrons from atoms are...
A)shared
B)transferred to the less metallic element
C)transferred to the more metallic element
D)gained by the anion
E)gained by the cation
A)shared
B)transferred to the less metallic element
C)transferred to the more metallic element
D)gained by the anion
E)gained by the cation
shared
4
Consider the compound represented below. 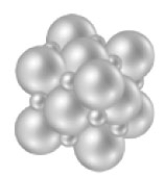 What is most likely the physical state of this substance at room temperature?
What is most likely the physical state of this substance at room temperature?
A)Liquid
B)Gas
C)Solid
D)Plasma
 What is most likely the physical state of this substance at room temperature?
What is most likely the physical state of this substance at room temperature?A)Liquid
B)Gas
C)Solid
D)Plasma

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
5
What is the electron configuration of an ion that is isoelectronic with neon?
A)[Ne]3s23p6
B)[Ar]3s23p6
C)[Ar]4s2
D)1s22s22p6
E)1s22s22p2
A)[Ne]3s23p6
B)[Ar]3s23p6
C)[Ar]4s2
D)1s22s22p6
E)1s22s22p2

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
6
A Lewis formula or Lewis diagram is used to show what?
A)The physical properties of the compound
B)How Lewisite can be made in the laboratory
C)Whether a bond is polar or nonpolar
D)How metals form alloys
E)The arrangement of atoms and electrons in a molecule
A)The physical properties of the compound
B)How Lewisite can be made in the laboratory
C)Whether a bond is polar or nonpolar
D)How metals form alloys
E)The arrangement of atoms and electrons in a molecule

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
7
Which of the following has/have the electron configuration [Ne]3s23p6?
(i)S2-
(ii)Cl
(iii)Ar
(iv)K+
(v)Ca2+
A)i only
B)iv and v
C)ii and iii
D)i,iii,iv,and v
E)All have the electron configuration [Ne]3s23p6
(i)S2-
(ii)Cl
(iii)Ar
(iv)K+
(v)Ca2+
A)i only
B)iv and v
C)ii and iii
D)i,iii,iv,and v
E)All have the electron configuration [Ne]3s23p6

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
8
How does a phosphorus atom achieve an octet of electrons?
A)By losing 5 electrons
B)By losing 3 electrons
C)By gaining 8 electrons
D)By gaining 5 electrons
E)By gaining 3 electrons
A)By losing 5 electrons
B)By losing 3 electrons
C)By gaining 8 electrons
D)By gaining 5 electrons
E)By gaining 3 electrons

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
9
Consider the following periodic table. 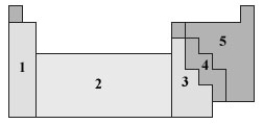 In which of the numbered sections will elements form anions that are isoelectronic with a noble gas?
In which of the numbered sections will elements form anions that are isoelectronic with a noble gas?
A)1
B)2
C)3
D)4
E)5
 In which of the numbered sections will elements form anions that are isoelectronic with a noble gas?
In which of the numbered sections will elements form anions that are isoelectronic with a noble gas?A)1
B)2
C)3
D)4
E)5

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
10
What is a lone pair in a Lewis diagram?
A)A bonding pair of electrons
B)A shared pair of electrons
C)A molecule with only two electrons
D)An unshared pair of electrons
E)The electrons that are not counted when determining if the octet of an atom has been satisfied
A)A bonding pair of electrons
B)A shared pair of electrons
C)A molecule with only two electrons
D)An unshared pair of electrons
E)The electrons that are not counted when determining if the octet of an atom has been satisfied

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
11
Why does the stability of a noble gas electron configuration contribute to the formation of covalent bonds?
A)Ionic bonds are called electron transfer bonds and covalent bonds are called electron sharing bonds
B)Noble gases are found in Group 8A/18 on the periodic table
C)Electrons occur in pairs in covalent bonds
D)Ionic crystals are held together by electrostatic forces and molecular compounds are not
E)The tendency toward a complete octet of electrons in a bonded atom reflects the natural tendency for a system to move to the lowest energy state possible
A)Ionic bonds are called electron transfer bonds and covalent bonds are called electron sharing bonds
B)Noble gases are found in Group 8A/18 on the periodic table
C)Electrons occur in pairs in covalent bonds
D)Ionic crystals are held together by electrostatic forces and molecular compounds are not
E)The tendency toward a complete octet of electrons in a bonded atom reflects the natural tendency for a system to move to the lowest energy state possible

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
12
The general term to indicate a negatively charged ion is ...
A)a cation
B)an anion
C)a hydrated ion
D)isoelectron
E)a valence ion
A)a cation
B)an anion
C)a hydrated ion
D)isoelectron
E)a valence ion

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
13
How is the bond in an F2 molecule formed?
A)Overlap of the half-filled 1s orbitals of two fluorine atoms
B)Overlap of the half-filled 1s orbital from one fluorine atom with the half-filled 2p orbital from a second fluorine atom
C)Overlap of the half-filled 2p orbitals of two fluorine atoms
D)Transfer of an electron from one fluorine atom to the other
E)Transfer of an electron from one fluorine atom to third atom,followed by an electron from a neutral fluorine atom being shared with the electron-deficient fluorine atom to form the bond
A)Overlap of the half-filled 1s orbitals of two fluorine atoms
B)Overlap of the half-filled 1s orbital from one fluorine atom with the half-filled 2p orbital from a second fluorine atom
C)Overlap of the half-filled 2p orbitals of two fluorine atoms
D)Transfer of an electron from one fluorine atom to the other
E)Transfer of an electron from one fluorine atom to third atom,followed by an electron from a neutral fluorine atom being shared with the electron-deficient fluorine atom to form the bond

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
14
Which of the following ions is/are isoelectronic with Kr?
(i)Cl-
(ii)Br-
(iii)K+
(iv)Rb+
(v)Kr4+
A)v only
B)i and ii
C)ii and iv
D)iii and iv
E)iii,iv,and v
(i)Cl-
(ii)Br-
(iii)K+
(iv)Rb+
(v)Kr4+
A)v only
B)i and ii
C)ii and iv
D)iii and iv
E)iii,iv,and v

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
15
Which of the following correctly describes a covalent bond?
A)The charge clouds of the 1s orbitals repel one another
B)The charge density of the electrons is concentrated between the two nuclei
C)The electron clouds do not overlap as much as in an ionic bond
D)All the valence electrons go into the electron cloud of only one of the atoms
E)There are no valence electrons
A)The charge clouds of the 1s orbitals repel one another
B)The charge density of the electrons is concentrated between the two nuclei
C)The electron clouds do not overlap as much as in an ionic bond
D)All the valence electrons go into the electron cloud of only one of the atoms
E)There are no valence electrons

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
16
Which of these ions has a Lewis symbol of the following form? 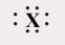
A)N3-
B)F+
C)C4+
D)Ar
E)O22-
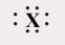
A)N3-
B)F+
C)C4+
D)Ar
E)O22-

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
17
Which of the following correctly describes a cation?
A)A positively charged ion
B)An ion formed by the addition of electrons to the neutral atom
C)An ion formed mostly by the nonmetals
D)An ion that contains a greater number of electrons than protons
E)An ion formed by elements on the right side of the periodic table
A)A positively charged ion
B)An ion formed by the addition of electrons to the neutral atom
C)An ion formed mostly by the nonmetals
D)An ion that contains a greater number of electrons than protons
E)An ion formed by elements on the right side of the periodic table

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
18
The ratio of anions to cations in an ionic compound is always such that...
A)the compound is reduced in size when compared to the parent atoms
B)there are as many anions as there are cations
C)the anions outnumber the cations
D)the cations outnumber the anions
E)the compound is electrically neutral
A)the compound is reduced in size when compared to the parent atoms
B)there are as many anions as there are cations
C)the anions outnumber the cations
D)the cations outnumber the anions
E)the compound is electrically neutral

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
19
Which of the following statements correctly describe ionic compounds?
(i)They never contain covalent bonds
(ii)They are formed when atoms share their valence electrons
(iii)They are usually solids at room temperature
(iv)They are generally gases at ice water temperature
A)iii only
B)i and ii
C)i and iii
D)i and iv
E)i,ii,and iii
(i)They never contain covalent bonds
(ii)They are formed when atoms share their valence electrons
(iii)They are usually solids at room temperature
(iv)They are generally gases at ice water temperature
A)iii only
B)i and ii
C)i and iii
D)i and iv
E)i,ii,and iii

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
20
When a covalent bond forms,the atomic orbitals of the separated atoms...
A)repel one another
B)change from s orbitals to p orbitals
C)change from p,d,or f orbitals to s orbitals
D)cease to exist because the electrons transfer out of the orbitals
E)overlap
A)repel one another
B)change from s orbitals to p orbitals
C)change from p,d,or f orbitals to s orbitals
D)cease to exist because the electrons transfer out of the orbitals
E)overlap

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
21
Given the following electronegativities: C = 2.5,N = 3.0,O = 3.5,F = 4.0,arrange the bonds according to decreasing polarity.
A)C-O > C-N > C-C > C-F
B)C-F > C-C > C-N > C-O
C)C-F > C-O > C-N > C-C
D)C-C > C-N > C-O > C-F
E)C-N > C-O > C-F > C-C
A)C-O > C-N > C-C > C-F
B)C-F > C-C > C-N > C-O
C)C-F > C-O > C-N > C-C
D)C-C > C-N > C-O > C-F
E)C-N > C-O > C-F > C-C

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
22
Arrange the following bonds in order of increasing polarity.
Element
Electronegativity
C
2)5
P
2)1
As
2)0
S
2)5
Se
2)4
A)C-P < C-As < C-S < C-Se
B)C-Se < C-P < C-As < C-S
C)C-S < C-As < C-P < C- Se
D)C-As < C-P < C-Se < C-S
E)C-S < C-Se < C-P < C-As
Element
Electronegativity
C
2)5
P
2)1
As
2)0
S
2)5
Se
2)4
A)C-P < C-As < C-S < C-Se
B)C-Se < C-P < C-As < C-S
C)C-S < C-As < C-P < C- Se
D)C-As < C-P < C-Se < C-S
E)C-S < C-Se < C-P < C-As

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
23
Which of the following terms best describes the distribution of bonding electron charge in a polar bond?
A)Linear
B)Ordered
C)Random
D)Asymmetrical
E)Artificial
A)Linear
B)Ordered
C)Random
D)Asymmetrical
E)Artificial

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
24
Arrange the following bonds in order of increasing polarity.
Element
Electronegativity
H
2)1
N
3)0
O
3)5
P
2)1
S
2)5
A)H-O < H-N < H-S < H-P
B)H-P < H-S < H-N < H-O
C)H-N < H-O < H-P < H-S
D)H-S < H-P < H-O < H-N
E)H-P < H-S < H-O < H-N
Element
Electronegativity
H
2)1
N
3)0
O
3)5
P
2)1
S
2)5
A)H-O < H-N < H-S < H-P
B)H-P < H-S < H-N < H-O
C)H-N < H-O < H-P < H-S
D)H-S < H-P < H-O < H-N
E)H-P < H-S < H-O < H-N

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
25
Which of the following chemical bonds is best described as nonpolar covalent?
A)H-H
B)H-C
C)H-N
D)H-O
E)H-F
A)H-H
B)H-C
C)H-N
D)H-O
E)H-F

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
26
Consider the periodic table shown below
)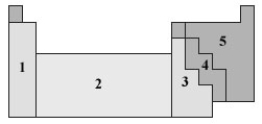
In which of the numbered regions will elements be found that have low electronegativity values?
A)1
B)2
C)3
D)4
E)5
)

In which of the numbered regions will elements be found that have low electronegativity values?
A)1
B)2
C)3
D)4
E)5

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
27
Which of the following is the best classification for a bond in which bonding electrons are shared equally?
A)Nonpolar
B)Polar covalent
C)Primarily ionic
D)Very strongly polar covalent
E)Slightly ionic
A)Nonpolar
B)Polar covalent
C)Primarily ionic
D)Very strongly polar covalent
E)Slightly ionic

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
28
Given the following electronegativities: H = 2.1,F = 4.0,C = 2.5,Cl = 3.0,arrange the bonds according to increasing polarity.
A)H-C < H-Cl < H-F < C-Cl
B)H-F < H-Cl < C-Cl < H-C
C)H-C < C-Cl < H-Cl < H-F
D)H-F < C-Cl < H-Cl < H-C
E)H-Cl < H-C < H-F < C-Cl
A)H-C < H-Cl < H-F < C-Cl
B)H-F < H-Cl < C-Cl < H-C
C)H-C < C-Cl < H-Cl < H-F
D)H-F < C-Cl < H-Cl < H-C
E)H-Cl < H-C < H-F < C-Cl

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
29
Among the following bonds,which would be the least polar? Electronegativity values are: Na = 0.9,O = 3.5,F = 4.0,Cl = 3.0,Br = 2.8,I = 2.5
A)Na-O
B)Na-F
C)Na-Cl
D)Na-Br
E)Na-I
A)Na-O
B)Na-F
C)Na-Cl
D)Na-Br
E)Na-I

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
30
Which of the following correctly lists the bonds with positive poles on the left and negative poles on the right?
+ - + - + -
A)I-Br I-Cl Cl-Br
B)I-Br I-Cl Br-Cl
C)I-Br Cl-I Cl-Br
D)I-Br Cl-I Br-Cl
E)Br-I Cl-I Cl-Br
+ - + - + -
A)I-Br I-Cl Cl-Br
B)I-Br I-Cl Br-Cl
C)I-Br Cl-I Cl-Br
D)I-Br Cl-I Br-Cl
E)Br-I Cl-I Cl-Br

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
31
Which element will act as the negative pole in each of the following bonds:
Element
Electronegativity
O
3)5
F
4)0
Cl
3)0
A)F in O-F and O in O-Cl
B)O in O-F and O in O-Cl
C)F in O-F and Cl in O-Cl
D)O in O-F and Cl in O-Cl
E)O,F,and Cl are all highly electronegative so there will be neither a positive nor a negative pole.
Element
Electronegativity
O
3)5
F
4)0
Cl
3)0
A)F in O-F and O in O-Cl
B)O in O-F and O in O-Cl
C)F in O-F and Cl in O-Cl
D)O in O-F and Cl in O-Cl
E)O,F,and Cl are all highly electronegative so there will be neither a positive nor a negative pole.

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
32
Which of the following is the best classification for a bond in which bonding electrons are shared unequally?
A)Nonpolar covalent
B)Polar covalent
C)Electronegative
D)Multiple
E)Completely ionic
A)Nonpolar covalent
B)Polar covalent
C)Electronegative
D)Multiple
E)Completely ionic

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
33
How many single,double,and triple bonds are in the following molecule? 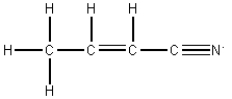 # single bonds # double bonds # triple bonds
# single bonds # double bonds # triple bonds
A)5 1 1
B)7 1 1
C)8 1 1
D)9 1 1
E)10 1 0
 # single bonds # double bonds # triple bonds
# single bonds # double bonds # triple bondsA)5 1 1
B)7 1 1
C)8 1 1
D)9 1 1
E)10 1 0

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
34
Consider the following image of two atoms interacting.Each small dot represents an instantaneous position of an electron and the circles represent the nuclei.  Which of the following is the best description of the type bond represented?
Which of the following is the best description of the type bond represented?
A)Nonpolar ionic
B)Nonpolar covalent
C)Polar ionic
D)Polar covalent
E)Metallic
 Which of the following is the best description of the type bond represented?
Which of the following is the best description of the type bond represented?A)Nonpolar ionic
B)Nonpolar covalent
C)Polar ionic
D)Polar covalent
E)Metallic

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
35
Which bond is completely nonpolar?
A)F-F
B)H-F
C)C-N
D)H-O
E)C-H
A)F-F
B)H-F
C)C-N
D)H-O
E)C-H

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
36
In deciding whether a bond is polar or nonpolar,what has to be considered?
A)The electromagnetic field surrounding the molecule
B)Whether a magnet will attract the bonded atoms
C)The electronegativity difference between the atoms
D)The number of lone pairs
E)The number of bonds between the two atoms
A)The electromagnetic field surrounding the molecule
B)Whether a magnet will attract the bonded atoms
C)The electronegativity difference between the atoms
D)The number of lone pairs
E)The number of bonds between the two atoms

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
37
Which element will act as the negative pole in each of the following bonds: Li-H,H-C?
Element Electronegativity
Li 1.0
H 2.1
C 2.5
A)Li in Li-H and C in H-C
B)Li in Li-H and H in H-C
C)H in Li-H and C in H-C
D)H in Li-H and H in H-C
E)The bonds in H-C are not polar and cannot be classified as having a negative pole.
Element Electronegativity
Li 1.0
H 2.1
C 2.5
A)Li in Li-H and C in H-C
B)Li in Li-H and H in H-C
C)H in Li-H and C in H-C
D)H in Li-H and H in H-C
E)The bonds in H-C are not polar and cannot be classified as having a negative pole.

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
38
Which is the correct procedure to rank bonds in order of increasing polarity?
A)List them from least electronegative nonmetal to most electronegative nonmetal
B)List them from most electronegative nonmetal to least electronegative nonmetal
C)List them from least electronegativity difference between elements to greatest electronegativity difference between elements
D)List them from greatest electronegativity difference between elements to least electronegativity difference between elements
E)List them from least total number of unshared electron pairs to greatest total number of unshared electron pairs
A)List them from least electronegative nonmetal to most electronegative nonmetal
B)List them from most electronegative nonmetal to least electronegative nonmetal
C)List them from least electronegativity difference between elements to greatest electronegativity difference between elements
D)List them from greatest electronegativity difference between elements to least electronegativity difference between elements
E)List them from least total number of unshared electron pairs to greatest total number of unshared electron pairs

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
39
Which of the following statements is incorrect?
A)Electronegativities tend to be greater at the top of any group
B)Electronegativities increase from left to right across any row of the periodic table
C)You can estimate the polarity of a bond by calculating the difference between the electronegativity values for the two elements
D)The electronegativity difference is zero between elements with electrons in the same principal energy level
E)The more electronegative element toward which the bonding electrons are displaced acts as the "negative pole" in a polar bond
A)Electronegativities tend to be greater at the top of any group
B)Electronegativities increase from left to right across any row of the periodic table
C)You can estimate the polarity of a bond by calculating the difference between the electronegativity values for the two elements
D)The electronegativity difference is zero between elements with electrons in the same principal energy level
E)The more electronegative element toward which the bonding electrons are displaced acts as the "negative pole" in a polar bond

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
40
How many electrons are in a single,double,and triple bond,respectively?
Single double triple
A)1 2 3
B)2 4 6
C)3 6 9
D)4 8 12
E)5 10 15
Single double triple
A)1 2 3
B)2 4 6
C)3 6 9
D)4 8 12
E)5 10 15

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
41
Examine the following model. 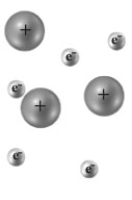 What type of bonding does this model represent?
What type of bonding does this model represent?
A)Polar
B)Nonpolar
C)Ionic
D)Covalent
E)Metallic
 What type of bonding does this model represent?
What type of bonding does this model represent?A)Polar
B)Nonpolar
C)Ionic
D)Covalent
E)Metallic

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
42
If you were to sketch a particulate-level illustration of the electron-sea model of metallic bonding for calcium,and your sketch showed eight calcium nuclei and their associated electrons,how many "free" electrons should also be in your sketch?
A)2
B)4
C)8
D)16
E)more than 16
A)2
B)4
C)8
D)16
E)more than 16

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
43
Which of the following correctly describes the bonding in ionic,covalent,and metallic bonds?
Ionic covalent metallic
A)localized localized localized
B)delocalized localized localized
C)localized localized delocalized
D)localized delocalized localized
E)delocalized delocalized delocalized
Ionic covalent metallic
A)localized localized localized
B)delocalized localized localized
C)localized localized delocalized
D)localized delocalized localized
E)delocalized delocalized delocalized

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
44
Match each of the following terms with the appropriate bonding type.
Electron donating Electron sharing Delocalized electrons
A)Metallic Covalent Ionic
B)Metallic Ionic Covalent
C)Covalent Ionic Metallic
D)Ionic Metallic Covalent
E)Ionic Covalent Metallic
Electron donating Electron sharing Delocalized electrons
A)Metallic Covalent Ionic
B)Metallic Ionic Covalent
C)Covalent Ionic Metallic
D)Ionic Metallic Covalent
E)Ionic Covalent Metallic

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
45
Which of the following is the best description of metallic bonding?
A)Positively charged metal ions surrounded by freely moving electrons
B)Alternating positively charged metal ions and negatively charged metal ions
C)Pairs of metal atoms sharing electrons,plus weak intermolecular forces holding the pairs in place in the metal crystal
D)Groups of three metal atoms sharing electrons (three-center bonds),plus weak intermolecular forces holding the groups in place in the metal crystal
E)Metallic bonding is essentially the same as covalent bonding,except that in metallic bonding,protons are shared rather than electrons
A)Positively charged metal ions surrounded by freely moving electrons
B)Alternating positively charged metal ions and negatively charged metal ions
C)Pairs of metal atoms sharing electrons,plus weak intermolecular forces holding the pairs in place in the metal crystal
D)Groups of three metal atoms sharing electrons (three-center bonds),plus weak intermolecular forces holding the groups in place in the metal crystal
E)Metallic bonding is essentially the same as covalent bonding,except that in metallic bonding,protons are shared rather than electrons

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck



