Deck 20: Nuclear Chemistry
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/50
Play
Full screen (f)
Deck 20: Nuclear Chemistry
1
Which of the following is not an instrument that can be used to detect radioactivity?
(i)Photographic film
(ii)Gamma camera and scanner
(iii)Geiger counter
(iv)Scintillation counter
A)i only
B)iv only
C)i and iv
D)ii and iii
E)All can be used to detect radioactivity
(i)Photographic film
(ii)Gamma camera and scanner
(iii)Geiger counter
(iv)Scintillation counter
A)i only
B)iv only
C)i and iv
D)ii and iii
E)All can be used to detect radioactivity
All can be used to detect radioactivity
2
Consider the following image representing an atom. 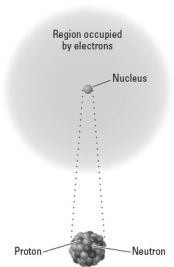 Which of the following types of radioactive decay would produce the largest mass change in the structure shown at the bottom of the image?
Which of the following types of radioactive decay would produce the largest mass change in the structure shown at the bottom of the image?
A)" "
B)" "
C)" "
D)" " and " " would produce about the same change.
 Which of the following types of radioactive decay would produce the largest mass change in the structure shown at the bottom of the image?
Which of the following types of radioactive decay would produce the largest mass change in the structure shown at the bottom of the image?A)" "
B)" "
C)" "
D)" " and " " would produce about the same change.
" "
3
A sample of 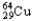 has an original mass of 57.3 mg,and after 24.0 hours the sample mass is 15.6 mg.1.88 half-lives correspond to 27.2% of the sample remaining.What is the half-life of
has an original mass of 57.3 mg,and after 24.0 hours the sample mass is 15.6 mg.1.88 half-lives correspond to 27.2% of the sample remaining.What is the half-life of 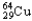
?
A)6.53 hr
B)12.3 hr
C)12.8 hr
D)24.0 hr
E)45.1 hr
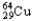 has an original mass of 57.3 mg,and after 24.0 hours the sample mass is 15.6 mg.1.88 half-lives correspond to 27.2% of the sample remaining.What is the half-life of
has an original mass of 57.3 mg,and after 24.0 hours the sample mass is 15.6 mg.1.88 half-lives correspond to 27.2% of the sample remaining.What is the half-life of 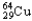
?
A)6.53 hr
B)12.3 hr
C)12.8 hr
D)24.0 hr
E)45.1 hr
12.8 hr
4
The half-life of 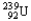 is 24 minutes.A freshly prepared sample is allowed to sit for 2.0 hours.If the mass of the sample after this period of time is 1.0 mg,what was the original sample mass?
is 24 minutes.A freshly prepared sample is allowed to sit for 2.0 hours.If the mass of the sample after this period of time is 1.0 mg,what was the original sample mass?
A)0.031 mg
B)1.3 mg
C)5.0 mg
D)32 mg
E)3.2* 102 mg
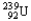 is 24 minutes.A freshly prepared sample is allowed to sit for 2.0 hours.If the mass of the sample after this period of time is 1.0 mg,what was the original sample mass?
is 24 minutes.A freshly prepared sample is allowed to sit for 2.0 hours.If the mass of the sample after this period of time is 1.0 mg,what was the original sample mass?A)0.031 mg
B)1.3 mg
C)5.0 mg
D)32 mg
E)3.2* 102 mg

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
5
Which of the following lists the three principal forms of radioactive emission in order of decreasing mass?
A)gamma > alpha > beta
B)gamma > beta > alpha
C)alpha > beta > gamma
D)alpha > gamma > beta
E)beta > gamma > alpha
A)gamma > alpha > beta
B)gamma > beta > alpha
C)alpha > beta > gamma
D)alpha > gamma > beta
E)beta > gamma > alpha

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
6
Consider the following description of an instrument used to detect radioactivity:
A tube contains argon at low pressure.A high electrical potential is established between a positively charged wire in the center and the negatively charged case.Radiation enters the window and ionizes the argon.The ions move toward the electrodes,producing a measurable electrical pulse and an audible "click." What is the name of this instrument?
A)Gamma camera and scanner
B)Scintillation counter
C)Geiger counter
D)Cloud chamber
E)Spectrophotometer
A tube contains argon at low pressure.A high electrical potential is established between a positively charged wire in the center and the negatively charged case.Radiation enters the window and ionizes the argon.The ions move toward the electrodes,producing a measurable electrical pulse and an audible "click." What is the name of this instrument?
A)Gamma camera and scanner
B)Scintillation counter
C)Geiger counter
D)Cloud chamber
E)Spectrophotometer

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
7
Which of the following lists the three principal forms of radioactive emission in order of increasing penetrating power?
A)alpha < beta < gamma
B)alpha < gammaC)beta < alpha > gamma
D)gamma < beta < alpha
E)gamma < alpha < beta
A)alpha < beta < gamma
B)alpha < gamma
D)gamma < beta < alpha
E)gamma < alpha < beta

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
8
Which of the following is the estimated average annual exposure to naturally occurring radiation sources?
A)3,600 mrem/yr
B)3.6 mrem/yr
C)0.360 mrem/yr
D)360 mrem/yr
E)36 mrem/yr
A)3,600 mrem/yr
B)3.6 mrem/yr
C)0.360 mrem/yr
D)360 mrem/yr
E)36 mrem/yr

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
9
To describe quantitatively the effects of radiation on a living system you must measure which of the following?
(i)The number of disintegrations that occur during the time of exposure
(ii)The amount of energy released with each disintegration
(iii)The amount of energy absorbed by the living system
A)ii only
B)iii only
C)i and ii
D)ii and iii
E)i,ii,and iii
(i)The number of disintegrations that occur during the time of exposure
(ii)The amount of energy released with each disintegration
(iii)The amount of energy absorbed by the living system
A)ii only
B)iii only
C)i and ii
D)ii and iii
E)i,ii,and iii

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
10
An ancient tree is found which has a carbon-14 disintegration rate of 11.3 units.If the disintegration rate of a living tree is 14.0 units,calculate the age of the ancient tree.t1/2 = 5.73 * 103 years for carbon-14 and 0.807 of the sample remaining corresponds to 0.309 half-life.
A)1.77 * 103 yr
B)4.62 * 103 yr
C)5.73 *103 yr
D)1.85 * 104 yr
E)7.10 * 104 yr
A)1.77 * 103 yr
B)4.62 * 103 yr
C)5.73 *103 yr
D)1.85 * 104 yr
E)7.10 * 104 yr

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
11
Which of the following is not a source of radiation?
(i)medical X rays
(ii)tobacco smoke
(iii)nuclear medicine
A)i only
B)i and ii
C)i and iii
D)ii and iii
E)All are sources of radiation
(i)medical X rays
(ii)tobacco smoke
(iii)nuclear medicine
A)i only
B)i and ii
C)i and iii
D)ii and iii
E)All are sources of radiation

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
12
Which of the following correctly matches the three principal forms of radioactive emission with their respective electrical charges?
A) " " has no charge, " " is -, " " is +
B)" " is -, " " has no charge," " is +
C) " " is -," " is +," " has no charge
D)" " is +," " has no charge," " is -
E)" " is +," " is -," " has no charge
A) " " has no charge, " " is -, " " is +
B)" " is -, " " has no charge," " is +
C) " " is -," " is +," " has no charge
D)" " is +," " has no charge," " is -
E)" " is +," " is -," " has no charge

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
13
Which of the following statements best summarizes the process that occurs which allows the detection of radioactivity?
A)A collision with radiation changes the proton arrangement in the target particle
B)A collision with the radiation changes the electron arrangement in the target particle
C)A collision with the radiation changes the neutron arrangement in the target particle
D)A collision with radiation changes the number of protons in the target particle
E)A collision with the radiation changes the number of neutrons in the target particle
A)A collision with radiation changes the proton arrangement in the target particle
B)A collision with the radiation changes the electron arrangement in the target particle
C)A collision with the radiation changes the neutron arrangement in the target particle
D)A collision with radiation changes the number of protons in the target particle
E)A collision with the radiation changes the number of neutrons in the target particle

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
14
Which of the following statements is or are false?
(i)Radioactivity first appeared on earth in the late 1800s.
(ii)Atoms cannot be changed from one element to another.
(iii)If a sample of a substance is radioactive,the sample remains radioactive forever.
A)i only
B)i and ii
C)i and iii
D)ii and iii
E)i,ii,and iii
(i)Radioactivity first appeared on earth in the late 1800s.
(ii)Atoms cannot be changed from one element to another.
(iii)If a sample of a substance is radioactive,the sample remains radioactive forever.
A)i only
B)i and ii
C)i and iii
D)ii and iii
E)i,ii,and iii

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
15
Which of the following is the best definition of radioactivity?
A)The logarithmic relationship between the logarithm of the activity coefficient and the radiation emitted by a radioactive nucleus.
B)The relative activity of the nuclear properties of electromagnetic radiation in the radio wave frequencies of the electromagnetic spectrum.
C)The spontaneous emission of particles and/or electromagnetic radiation resulting from the decay of an atomic nucleus.
D)The emission of electrons from an ionic compound that has unbalanced charge,after being stimulated by the radiation from the radio wave frequencies of the electromagnetic spectrum.
E)The unnatural radiation emitted by manmade nuclear processes such as nuclear power plants and atomic bombs.
A)The logarithmic relationship between the logarithm of the activity coefficient and the radiation emitted by a radioactive nucleus.
B)The relative activity of the nuclear properties of electromagnetic radiation in the radio wave frequencies of the electromagnetic spectrum.
C)The spontaneous emission of particles and/or electromagnetic radiation resulting from the decay of an atomic nucleus.
D)The emission of electrons from an ionic compound that has unbalanced charge,after being stimulated by the radiation from the radio wave frequencies of the electromagnetic spectrum.
E)The unnatural radiation emitted by manmade nuclear processes such as nuclear power plants and atomic bombs.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
16
A waste sample exhibits radioactivity.In order the determine the total level of radioactivity of the sample,which of the following should be used?
A)Geiger counter
B)scintillation counter
C)gamma camera scanner
D)a combination of a Geiger and a scintillation counter.
A)Geiger counter
B)scintillation counter
C)gamma camera scanner
D)a combination of a Geiger and a scintillation counter.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
17
The half-life of 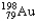 is 2.7 days.If you begin with 25 grams of
is 2.7 days.If you begin with 25 grams of 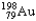
,how many grams will you have 10.8 days later?
A)1.6 g
B)2.3 g
C)6.3 g
D)9.3 g
E)0.32 g
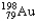 is 2.7 days.If you begin with 25 grams of
is 2.7 days.If you begin with 25 grams of 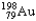
,how many grams will you have 10.8 days later?
A)1.6 g
B)2.3 g
C)6.3 g
D)9.3 g
E)0.32 g

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
18
Which of the following units is most commonly used to express radiation exposure?
A)rem
B)R
C)J
D)rad
E)All can be used to measure radiation exposure.
A)rem
B)R
C)J
D)rad
E)All can be used to measure radiation exposure.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
19
Which of the following is the best definition of the term half-life,as it applies to radioactive substances?
A)The time it takes for one half of the radioactive atoms in a sample to decay
B)Half of the time it takes for the radioactive atoms in a sample to decay
C)The number of radioactive atoms in a sample that decay in one half of a specified time period
D)The average lifetime of a radioactive atom
E)Half of the average lifetime of a radioactive atom
A)The time it takes for one half of the radioactive atoms in a sample to decay
B)Half of the time it takes for the radioactive atoms in a sample to decay
C)The number of radioactive atoms in a sample that decay in one half of a specified time period
D)The average lifetime of a radioactive atom
E)Half of the average lifetime of a radioactive atom

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
20
Which of the following is the best definition of the term transmutation?
A)Conversion of an atom from one element to another by means of a nuclear change
B)A change that appears to take place by itself without outside influence
C)The nucleus of a helium atom,often emitted in nuclear disintegration
D)To change chemically into simpler substances
E)A high-energy photon emission in radioactive disintegration
A)Conversion of an atom from one element to another by means of a nuclear change
B)A change that appears to take place by itself without outside influence
C)The nucleus of a helium atom,often emitted in nuclear disintegration
D)To change chemically into simpler substances
E)A high-energy photon emission in radioactive disintegration

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
21
Which of the following is the best description of a positron?
A)Positively charged electromagnetic radiation
B)A proton
C)A positive electron
D)A particle accelerator
E)An artificial element
A)Positively charged electromagnetic radiation
B)A proton
C)A positive electron
D)A particle accelerator
E)An artificial element

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
22
Choose the correct nuclear symbol for the missing species to complete the following nuclear bombardment equation: 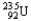 + ?
+ ? 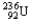
A)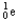
B)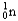
C)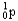
D)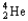
E)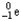
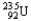 + ?
+ ? 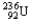
A)
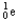
B)
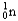
C)
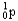
D)
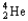
E)
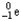

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
23
Which of the following best describes a natural radioactive decay series?
A)Change of one element to another by nuclear bombardment
B)A chain reaction,in which a nuclear product of one reaction becomes a nuclear reactant in the next step
C)The combination of two small nuclei to form a larger nucleus
D)A list of a series of reactions that illustrate how a radioactive element disintegrates into a stable isotope
E)A nuclear equation for a natural radioactive decay,showing the alpha and beta particles written as a series rather than with coefficients
A)Change of one element to another by nuclear bombardment
B)A chain reaction,in which a nuclear product of one reaction becomes a nuclear reactant in the next step
C)The combination of two small nuclei to form a larger nucleus
D)A list of a series of reactions that illustrate how a radioactive element disintegrates into a stable isotope
E)A nuclear equation for a natural radioactive decay,showing the alpha and beta particles written as a series rather than with coefficients

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
24
Which of the following represents the correct nuclear equation for the alpha decay of 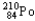 ?
?
A)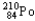
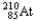
+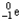
B)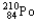 +
+ 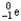
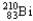
C)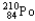
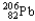 +
+ 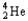
D) +
+ 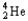
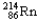
E)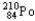
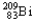 +
+ 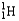
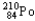 ?
?A)
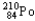
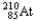
+
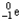
B)
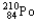 +
+ 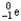
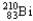
C)
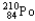
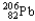 +
+ 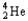
D)
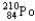 +
+ 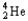
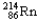
E)
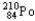
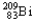 +
+ 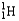

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
25
Which of the following represents the correct nuclear equation for the ejection of an alpha particle from 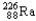 ?
?
A)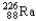
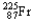 +
+ 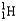
B)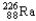
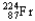 +
+ 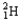
C)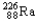
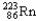 +
+ 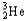
D)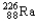
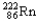 +
+ 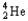
E)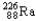
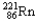 +
+ 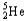
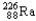 ?
?A)
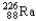
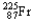 +
+ 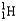
B)
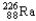
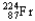 +
+ 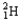
C)
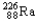
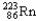 +
+ 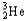
D)
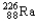
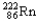 +
+ 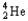
E)
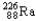
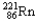 +
+ 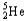

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
26
Choose the nuclear symbol for the missing species to complete the following nuclear bombardment equation: 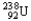 +
+ 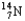
5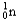
+ ?
A)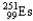
B)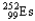
C)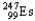
D)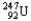
E)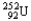
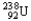 +
+ 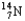
5
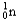
+ ?
A)
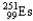
B)
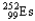
C)
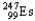
D)
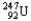
E)
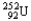

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
27
Which of the following statements is/are incorrect?
(i)Nuclear bombardment reactions produce isotopes that do not exist in nature
(ii)If a particle is to be used in a particle accelerator,it must have an electrical charge
(iii)All the elements having atomic numbers greater than 92 are called the transuranium elements
A)i only
B)i and ii
C)i and iii
D)All of the statements are incorrect
E)None of the statements is incorrect
(i)Nuclear bombardment reactions produce isotopes that do not exist in nature
(ii)If a particle is to be used in a particle accelerator,it must have an electrical charge
(iii)All the elements having atomic numbers greater than 92 are called the transuranium elements
A)i only
B)i and ii
C)i and iii
D)All of the statements are incorrect
E)None of the statements is incorrect

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
28
Which of the following is a process for which synthetic radioactive sample are used?
A)Medicinal applications
B)Food preservation
C)Studies of piston wear
D)Monitoring the progress of certain oils through pipelines
E)All of the above
A)Medicinal applications
B)Food preservation
C)Studies of piston wear
D)Monitoring the progress of certain oils through pipelines
E)All of the above

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
29
Which of the following statements is/are incorrect?
(i)Chemists use "tagged" atoms as radioactive tracers to study the series of individual steps in complicated reactions
(ii)A few dozen uses for synthetic radioactive nuclei are known today
(iii)Artificial radioactive nuclei cannot be used in food preservation because the food becomes radioactive
A)i only
B)iii only
C)i and ii
D)ii and iii
E)i,ii,and iii
(i)Chemists use "tagged" atoms as radioactive tracers to study the series of individual steps in complicated reactions
(ii)A few dozen uses for synthetic radioactive nuclei are known today
(iii)Artificial radioactive nuclei cannot be used in food preservation because the food becomes radioactive
A)i only
B)iii only
C)i and ii
D)ii and iii
E)i,ii,and iii

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
30
Which of the following is/are (a)transuranium element(s)?
(i)Pa (Z = 91)
(ii)U (Z = 92)
(iii)Np (Z = 93)
A)iii only
B)i and ii
C)ii and iii
D)None is a transuranium element
E)All are transuranium elements
(i)Pa (Z = 91)
(ii)U (Z = 92)
(iii)Np (Z = 93)
A)iii only
B)i and ii
C)ii and iii
D)None is a transuranium element
E)All are transuranium elements

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
31
Choose the correct nuclear symbol for the missing species to complete the following nuclear bombardment equation: 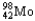 +
+ 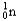
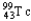
+ ?
A)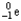
B)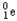
C)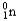
D)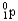
E)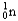
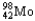 +
+ 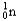
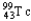
+ ?
A)
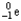
B)
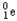
C)
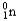
D)
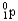
E)
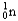

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
32
Which of the following is the best definition of the term nuclear fission?
A)A nuclear reaction in which two small nuclei combine to form a larger nucleus
B)A nuclear reaction in which a large nucleus splits into two smaller nuclei
C)The combination of a nucleus and an electron to produce an element with an atomic number one less than the original element
D)The combination of a nucleus and a positron to produce an element with an atomic number one more than the original element
E)The ejection of an electron from a nucleus to produce an element with an atomic number one more than the original element
A)A nuclear reaction in which two small nuclei combine to form a larger nucleus
B)A nuclear reaction in which a large nucleus splits into two smaller nuclei
C)The combination of a nucleus and an electron to produce an element with an atomic number one less than the original element
D)The combination of a nucleus and a positron to produce an element with an atomic number one more than the original element
E)The ejection of an electron from a nucleus to produce an element with an atomic number one more than the original element

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
33
Which of the following represents the correct nuclear equation for the beta emission of 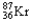 ?
?
A)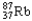 +
+ 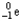
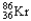
B)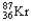
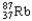 +
+ 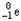
C)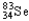 +
+ 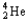
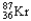
D)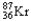
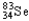 +
+ 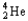
E)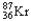 +
+ 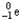
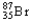
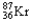 ?
?A)
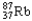 +
+ 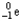
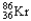
B)
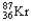
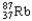 +
+ 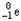
C)
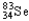 +
+ 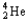
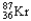
D)
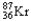
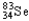 +
+ 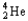
E)
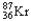 +
+ 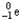
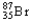

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
34
Which of the following statements best describes the relative amounts of energy for a given amount of reactant in ordinary chemical and nuclear reactions?
A)The chemical reaction is greater by a multiple less than 10
B)The nuclear reaction is greater by a multiple less than 10
C)The chemical reaction is greater by several multiples of 10
D)The nuclear reaction is greater by several multiples of 10
E)The chemical reaction is greater by more than 100,000
A)The chemical reaction is greater by a multiple less than 10
B)The nuclear reaction is greater by a multiple less than 10
C)The chemical reaction is greater by several multiples of 10
D)The nuclear reaction is greater by several multiples of 10
E)The chemical reaction is greater by more than 100,000

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
35
Which of the following statements is the best comparison of the chemical properties of oxygen-15 and oxygen-16?
A)The reactivity of the heavier elements will be greater with oxygen-16
B)The reactivity of the heavier elements will be greater with oxygen-15
C)Oxygen-16 will undergo more frequent reactions
D)Oxygen-15 will undergo more frequent reactions
E)There is no difference in the chemical properties of the two isotopes
A)The reactivity of the heavier elements will be greater with oxygen-16
B)The reactivity of the heavier elements will be greater with oxygen-15
C)Oxygen-16 will undergo more frequent reactions
D)Oxygen-15 will undergo more frequent reactions
E)There is no difference in the chemical properties of the two isotopes

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
36
Consider the following image representing an atom. 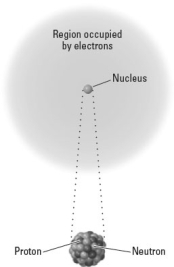 Which of the following would describe the net effect of the emission of a particles by this nucleus?
Which of the following would describe the net effect of the emission of a particles by this nucleus?
A)The number of protons would increase.
B)The number of neutrons would decrease.
C)The mass number of the product nucleus would the same as that shown.
D)The atomic number of the product nucleus would be one larger than that shown.
E)All of the above describe the net effect of this type of emission.
 Which of the following would describe the net effect of the emission of a particles by this nucleus?
Which of the following would describe the net effect of the emission of a particles by this nucleus?A)The number of protons would increase.
B)The number of neutrons would decrease.
C)The mass number of the product nucleus would the same as that shown.
D)The atomic number of the product nucleus would be one larger than that shown.
E)All of the above describe the net effect of this type of emission.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
37
Choose the correct nuclear symbol for the missing species to complete the following nuclear bombardment equation: 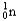 + ?
+ ? 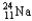
+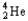
A)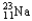
B)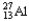
C)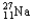
D)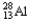
E)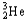
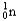 + ?
+ ? 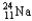
+
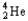
A)
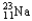
B)
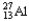
C)
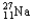
D)
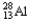
E)
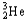

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
38
Which of the following equations is best classified as a nuclear fission reaction?
A)H+(aq)+ OH-(aq) H2O(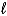 )
)
B)5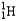 + 5
+ 5 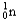
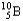
C)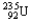 +
+ 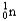
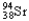 +
+ 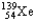 + 3
+ 3 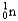
D)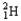 +
+ 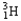
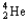 +
+ 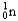
E)AgNO3(aq)+ NaCl(aq) NaNO3(aq)+ AgCl(s)
A)H+(aq)+ OH-(aq) H2O(
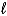 )
)B)5
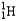 + 5
+ 5 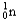
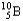
C)
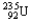 +
+ 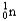
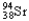 +
+ 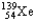 + 3
+ 3 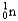
D)
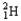 +
+ 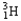
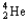 +
+ 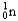
E)AgNO3(aq)+ NaCl(aq) NaNO3(aq)+ AgCl(s)

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
39
What distinguishes natural radioactivity from induced radioactivity produced by bombardment reactions?
A)Gamma rays are not emitted in natural radioactivity
B)Gamma rays are not emitted in induced radioactivity
C)Induced radioactivity produces radioactive elements not found in nature
D)Induced radioactivity is still a theoretical concept; natural radioactivity actually occurs
E)When naturally radioactive substances decay,they emit natural radiation,but when induced radioactive substances decay,they emit artificial radiation
A)Gamma rays are not emitted in natural radioactivity
B)Gamma rays are not emitted in induced radioactivity
C)Induced radioactivity produces radioactive elements not found in nature
D)Induced radioactivity is still a theoretical concept; natural radioactivity actually occurs
E)When naturally radioactive substances decay,they emit natural radiation,but when induced radioactive substances decay,they emit artificial radiation

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
40
Which of the following represents the correct nuclear equation for the beta decay of 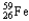 ?
?
A)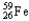
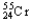 +
+ 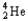
B)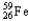
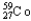 +
+ 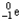
C)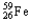 +
+ 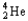
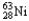
D)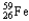 +
+ 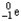
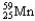
E)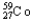 +
+ 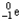
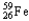
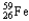 ?
?A)
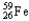
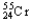 +
+ 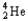
B)
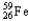
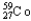 +
+ 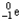
C)
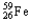 +
+ 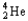
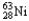
D)
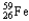 +
+ 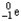
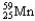
E)
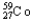 +
+ 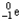
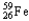

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
41
Which of the following best defines the term chain reaction?
A)A reaction that occurs in a star
B)A nuclear product of one reaction becomes a nuclear reactant in a subsequent reaction
C)A nuclear reaction that can have many products,each linked to the other like links in a chain
D)A reaction that occurs in fusion reactors in which the reactant nuclei are chemically chained to one another
E)A reaction that occurs in fusion reactors in which the product nuclei are chemically chained to one another
A)A reaction that occurs in a star
B)A nuclear product of one reaction becomes a nuclear reactant in a subsequent reaction
C)A nuclear reaction that can have many products,each linked to the other like links in a chain
D)A reaction that occurs in fusion reactors in which the reactant nuclei are chemically chained to one another
E)A reaction that occurs in fusion reactors in which the product nuclei are chemically chained to one another

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
42
Choose the three main components of a nuclear fission reactor from the following list:
(i)The fuel elements
(ii)The turbine
(iii)The generator
(iv)The control rods
(v)The moderator
(vi)The condenser
A)i,ii,and iv
B)i,iv,and v
C)ii,iii,and vi
D)iii,iv,and v
E)iii,iv,and vi
(i)The fuel elements
(ii)The turbine
(iii)The generator
(iv)The control rods
(v)The moderator
(vi)The condenser
A)i,ii,and iv
B)i,iv,and v
C)ii,iii,and vi
D)iii,iv,and v
E)iii,iv,and vi

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
43
Which of the following is the best description of the main obstacle to be overcome before energy can be obtained from fusion?
A)Safe storage and disposal of the radioactive waste
B)Radiation emitted from the plant itself
C)The extremely high temperature needed to start and sustain the reaction
D)A more abundant source of fuel
E)Better purification methods to separate the fuel
A)Safe storage and disposal of the radioactive waste
B)Radiation emitted from the plant itself
C)The extremely high temperature needed to start and sustain the reaction
D)A more abundant source of fuel
E)Better purification methods to separate the fuel

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
44
What is the most important difference between a nuclear power plant and a fossil-fueled power plant?
A)The nuclear power plant uses nuclear fuel and the fossil-fueled power plant uses fossil fuel,but the turbine,generator and condenser in both are similar.
B)The nuclear power plant uses nuclear fuel and the fossil-fueled power plant uses fossil fuel,and the turbine is not needed in a nuclear power plant,but the generator and condenser in both are similar.
C)The nuclear power plant uses nuclear fuel and the fossil-fueled power plant uses fossil fuel,and the turbine and generator are not needed in a nuclear power plant,but the condenser in both is similar.
D)The nuclear power plant uses nuclear fuel and the fossil-fueled power plant uses fossil fuel,but the turbine,generator,and condenser are not needed in a nuclear power plant.
E)The nuclear power plant uses nuclear fuel and the fossil-fueled power plant uses fossil fuel,but the turbine,generator,and condenser are not needed in a fossil-fueled power plant.
A)The nuclear power plant uses nuclear fuel and the fossil-fueled power plant uses fossil fuel,but the turbine,generator and condenser in both are similar.
B)The nuclear power plant uses nuclear fuel and the fossil-fueled power plant uses fossil fuel,and the turbine is not needed in a nuclear power plant,but the generator and condenser in both are similar.
C)The nuclear power plant uses nuclear fuel and the fossil-fueled power plant uses fossil fuel,and the turbine and generator are not needed in a nuclear power plant,but the condenser in both is similar.
D)The nuclear power plant uses nuclear fuel and the fossil-fueled power plant uses fossil fuel,but the turbine,generator,and condenser are not needed in a nuclear power plant.
E)The nuclear power plant uses nuclear fuel and the fossil-fueled power plant uses fossil fuel,but the turbine,generator,and condenser are not needed in a fossil-fueled power plant.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
45
Which of the following statements correctly compares fusion and fission reactions?
A)Fission reactions are harder to initiate than fusion reactions.
B)Fusion reactions tend to produce more energy than fission reactions.
C)Fission and fusion both produce larger nuclei as products.
D)Fission and fusion proceed via chain reactions.
A)Fission reactions are harder to initiate than fusion reactions.
B)Fusion reactions tend to produce more energy than fission reactions.
C)Fission and fusion both produce larger nuclei as products.
D)Fission and fusion proceed via chain reactions.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
46
Which of the following equations is best classified as a nuclear fusion reaction?
A)H+(aq)+ OH-(aq) H2O(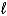 )
)
B)5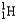 + 5
+ 5 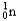
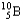
C)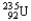 +
+ 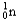
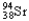 +
+ 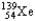 + 3
+ 3 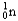
D)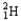 +
+ 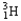
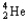 +
+ 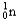
E)AgNO3(aq)+ NaCl(aq) NaNO3(aq)+ AgCl(s)
A)H+(aq)+ OH-(aq) H2O(
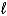 )
)B)5
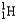 + 5
+ 5 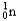
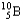
C)
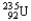 +
+ 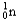
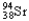 +
+ 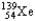 + 3
+ 3 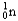
D)
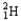 +
+ 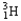
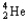 +
+ 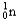
E)AgNO3(aq)+ NaCl(aq) NaNO3(aq)+ AgCl(s)

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
47
Which of the following statements is/are incorrect?
(i)The fuel elements in a nuclear fission reactor are simply long trays that hold fusionable matter in the reactor
(ii)The moderator in a nuclear fission reactor governs the reaction rate
(iii)The control rods in a nuclear fission reactor slow neutrons
A)i only
B)i and iii
C)ii and iii
D)None is incorrect
E)All are incorrect
(i)The fuel elements in a nuclear fission reactor are simply long trays that hold fusionable matter in the reactor
(ii)The moderator in a nuclear fission reactor governs the reaction rate
(iii)The control rods in a nuclear fission reactor slow neutrons
A)i only
B)i and iii
C)ii and iii
D)None is incorrect
E)All are incorrect

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
48
Which of the following correctly describes the use of nuclear fission for the production of electricity?
A)The United States has one of the largest percentages of electricity provided by nuclear fission.
B)European countries tend have the largest percentage of electricity provided by nuclear fission.
C)Only one or two nations world wide product more than 25% of electricity from nuclear fission.
D)Most countries of the Pacific rim produce at least 25% of electricity from nuclear fission.
A)The United States has one of the largest percentages of electricity provided by nuclear fission.
B)European countries tend have the largest percentage of electricity provided by nuclear fission.
C)Only one or two nations world wide product more than 25% of electricity from nuclear fission.
D)Most countries of the Pacific rim produce at least 25% of electricity from nuclear fission.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
49
Which of the following is or are reasons why the building and continued use of nuclear power plants faces some opposition in the United States?
(i)The threat of an accident that might release large amounts of radiation.
(ii)Disposal of the radioactive wastes that are generated by nuclear reactors.
(iii)Fear that an irresponsible government may use nuclear fuel to manufacture nuclear weapons.
A)iii only
B)i and ii
C)i and iii
D)ii and iii
E)i,ii,and iii
(i)The threat of an accident that might release large amounts of radiation.
(ii)Disposal of the radioactive wastes that are generated by nuclear reactors.
(iii)Fear that an irresponsible government may use nuclear fuel to manufacture nuclear weapons.
A)iii only
B)i and ii
C)i and iii
D)ii and iii
E)i,ii,and iii

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
50
Which of the following statements is/are correct?
(i)A chain reaction is a reaction that has as a product one of its own reactants
(ii)The critical mass of an element is the minimum mass necessary to react with a transuranium element
(iii)A breeder reactor is a device whose purpose is to produce fissionable fuel from nonfissionable isotopes
A)i and iii
B)ii and iii
C)i and ii
D)All are correct
E)None is correct
(i)A chain reaction is a reaction that has as a product one of its own reactants
(ii)The critical mass of an element is the minimum mass necessary to react with a transuranium element
(iii)A breeder reactor is a device whose purpose is to produce fissionable fuel from nonfissionable isotopes
A)i and iii
B)ii and iii
C)i and ii
D)All are correct
E)None is correct

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck



