Deck 20: Organic Chemistry
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/58
Play
Full screen (f)
Deck 20: Organic Chemistry
1
In an unsaturated hydrocarbon, the carbon-carbon bond angles are always 120o.
False
2
Carbon can form single and double bonds with other atoms, but not triple bonds.
False
3
Name the following molecule. 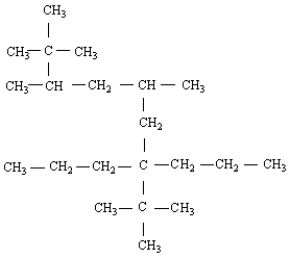
A) 2,2,3,5-tetramethyl-7-propyl-7-t-butyldecane
B) 6-propyl-2,6-di-t-butylnonane
C) 2,2,5,7,8,8-hexamethyl-3,3-dipropylnonane
D) isonanane
E) none of these

A) 2,2,3,5-tetramethyl-7-propyl-7-t-butyldecane
B) 6-propyl-2,6-di-t-butylnonane
C) 2,2,5,7,8,8-hexamethyl-3,3-dipropylnonane
D) isonanane
E) none of these
2,2,3,5-tetramethyl-7-propyl-7-t-butyldecane
4
A student gave a molecule the following name: 2-ethyl-3-methyl-5-isopropylhexane
However, her TA pointed out that although the molecule could be drawn correctly from this name, the name violates the systematic rules. What is the correct (systematic) name of the molecule?
A) 3,4-dimethyl-6-isopropylheptane
B) 2-isopropyl-4,5-dimethylheptane
C) 3,4,6,7-tetramethyloctane
D) 1,2-diethyl-3,6,7-trimethylheptane
E) 2,3,5,6-tetramethyloctane
However, her TA pointed out that although the molecule could be drawn correctly from this name, the name violates the systematic rules. What is the correct (systematic) name of the molecule?
A) 3,4-dimethyl-6-isopropylheptane
B) 2-isopropyl-4,5-dimethylheptane
C) 3,4,6,7-tetramethyloctane
D) 1,2-diethyl-3,6,7-trimethylheptane
E) 2,3,5,6-tetramethyloctane

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
5
What's the correct formula for the alkane that contains nine carbon atoms?
A) C9H20
B) C9H18
C) C9H16
D) C9H9
E) C9H11
A) C9H20
B) C9H18
C) C9H16
D) C9H9
E) C9H11

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
6
Name the following molecule. CH3-(CH2)2-CH3
A) butane
B) ethane
C) propane
D) benzene
E) pentane
A) butane
B) ethane
C) propane
D) benzene
E) pentane

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
7
Name the following molecule. 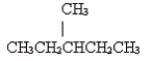
A) n-hexane
B) isohexane
C) 1,2,3-trimethylpropane
D) methyl-diethylmethane
E) 3-methylpentane
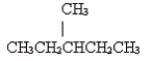
A) n-hexane
B) isohexane
C) 1,2,3-trimethylpropane
D) methyl-diethylmethane
E) 3-methylpentane

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
8
Benzene has the formula C6H6 and a planar (flat) structure in which all the bond angles are 109.5o.

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
9
What's the correct formula for the saturated hydrocarbon that contains four carbon atoms?
A) C4H10
B) C4H8
C) C4H12
D) C4H4
E) C4H6
A) C4H10
B) C4H8
C) C4H12
D) C4H4
E) C4H6

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
10
Name the following molecule. 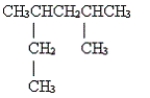


Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
11
In lecture, the professor named a molecule 2-ethyl-4-tertiary-butylpentane. An alert student pointed out that although the correct structure could be drawn from this name, the name did not follow systematic rules. What is the correct systematic name for the molecule?
A) 2-t-butyl-5-methylhexane
B) 2-ethyl-4,5,5-trimethylhexane
C) 3,5,6,6-tetramethylheptane
D) 2,2,3,5-tetramethylheptane
E) undecane
A) 2-t-butyl-5-methylhexane
B) 2-ethyl-4,5,5-trimethylhexane
C) 3,5,6,6-tetramethylheptane
D) 2,2,3,5-tetramethylheptane
E) undecane

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
12
Name the following molecule. 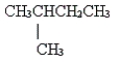
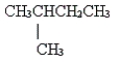

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
13
When carbon has four atoms bound to it, these atoms will have a square planar arrangement about the carbon.

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
14
Draw the structural formula for 2,2-dimethylpropane.

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
15
Those compounds whose carbon-carbon bonds are all single bonds are said to be unsaturated.

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
16
What's the correct formula for the simplest alkene that contains six carbon atoms?
A) C6H12
B) C6H10
C) C6H8
D) C6H14
E) C6H16
A) C6H12
B) C6H10
C) C6H8
D) C6H14
E) C6H16

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
17
A straight-chain saturated hydrocarbon cannot contain more than 10 carbon atoms.

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
18
Name the following molecule. 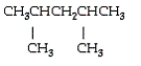
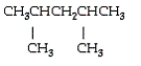

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
19
What's the correct formula for the simplest alkene that contains seven carbon atoms?
A) C7H14
B) C7H12
C) C7H10
D) C7H16
E) C7H18
A) C7H14
B) C7H12
C) C7H10
D) C7H16
E) C7H18

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
20
Butane is an example of an alkane.

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
21
Name the molecule CH3C CCH2CH3.

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
22
Give the name for the following molecule. 


Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
23
Name the following: 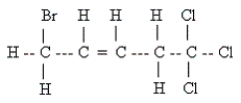
A) 1,1,1-trichloro-5-bromo-3-pentene
B) 5,5,5-trichloro-1-bromo-2-pentene
C) 1,1,1-trichloro-5-bromo-2-pentene
D) 1,1,1-trichloro-5-bromo-3-pentyne
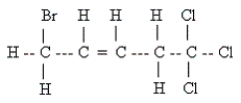
A) 1,1,1-trichloro-5-bromo-3-pentene
B) 5,5,5-trichloro-1-bromo-2-pentene
C) 1,1,1-trichloro-5-bromo-2-pentene
D) 1,1,1-trichloro-5-bromo-3-pentyne

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
24
Give the name of the molecule CH3CH=CHCH2CH3.

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
25
Name the molecule below. 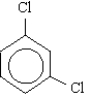


Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
26
Carboxylic acids are characterized by the presence of the -COOH group.

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
27
Name the following molecule. 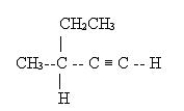
A) 1-hexyne
B) 2-ethynyl butane
C) 2-ethyl-3-butyne
D) 3-methyl-1-pentyne
E) 3-methyl-4-pentyne

A) 1-hexyne
B) 2-ethynyl butane
C) 2-ethyl-3-butyne
D) 3-methyl-1-pentyne
E) 3-methyl-4-pentyne

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
28
Draw the structural formula for 3-methyl-2-hexene.

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
29
Classify the following molecule. 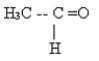
A) acid
B) aldehyde
C) amine
D) ketone
E) carbonyl
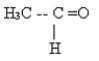
A) acid
B) aldehyde
C) amine
D) ketone
E) carbonyl

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
30
Draw the structural formula for 3-ethyl-4-methylheptane.

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
31
Which of the following has the least number of carbon-oxygen bonds?
A) ketone
B) alcohol
C) ether
D) ester
E) Two of the above are equal.
A) ketone
B) alcohol
C) ether
D) ester
E) Two of the above are equal.

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
32
The general name given to hydrocarbons with triple bonds is
A) alkenes
B) alkynes
C) alkanes
D) unsaturated hydrocarbons
E) aromatic hydrocarbons
A) alkenes
B) alkynes
C) alkanes
D) unsaturated hydrocarbons
E) aromatic hydrocarbons

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
33
Aldehydes are characterized by the presence of the -COH group (where carbon is double bonded to the oxygen atom and single bonded to the hydrogen).

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
34
Which of the following has the greatest number of carbon-oxygen bonds?
A) ketone
B) ester
C) alcohol
D) amine
E) aldehyde
A) ketone
B) ester
C) alcohol
D) amine
E) aldehyde

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
35
Give the name of the molecule. 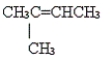
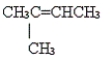

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
36
Amines are characterized by the presence of the -OH group.

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
37
Esters often have sweet, fruity odors.

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
38
CH3C CCH2CH2Cl is named
A) 1-chloro-3-pentyne
B) 5-chloro-2-pentene
C) 1-acetylenyl-3-chloropropane
D) 5-chloro-2-pentyne
E) 1-chloro-3-pentene
A) 1-chloro-3-pentyne
B) 5-chloro-2-pentene
C) 1-acetylenyl-3-chloropropane
D) 5-chloro-2-pentyne
E) 1-chloro-3-pentene

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
39
Draw the structural formula for 4-ethyl-2-octyne.

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
40
Draw the structural formula for 2-phenylpropane.

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
41
Acetic acid is the common name for ethanoic acid.

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
42
Name the following compound. 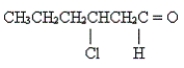
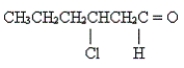

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
43
Draw the structural formula for pentanal.

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
44
Identify the type of organic compound shown. 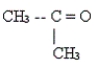
A) aldehyde
B) ester
C) amine
D) ketone
E) none of these
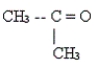
A) aldehyde
B) ester
C) amine
D) ketone
E) none of these

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
45
Write the structural formula for 3-isopropyl-3-heptanol.

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
46
Name the following compound. 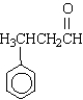


Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
47
Name the following compound. 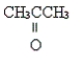
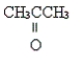

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
48
Which of the following is found in beverages such as wine?
A) methanol
B) ethanol
C) propanol
D) isopropanol
E) none of these
A) methanol
B) ethanol
C) propanol
D) isopropanol
E) none of these

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
49
Draw the structural formula for 3-ethylhexanoic acid.

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
50
Identify the type of organic compound shown. 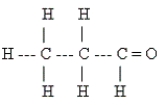
A) aldehyde
B) ester
C) amine
D) ketone
E) none of these
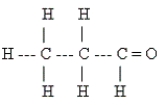
A) aldehyde
B) ester
C) amine
D) ketone
E) none of these

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
51
Nylon is an example of a
A) copolymer
B) homopolymer
C) dimer
D) two of these
E) none of these
A) copolymer
B) homopolymer
C) dimer
D) two of these
E) none of these

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
52
Draw the structural formula for 3,4-dichlorooctanal.

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
53
Name the following. 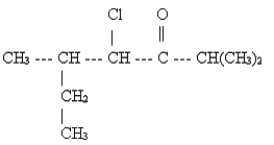
A) 2-chloro-3-ethyl-1-isopropylbutanone
B) isopropyl-chloromethylbutyl ketone
C) 2-butylchloroisobutanoyl methane
D) 4-chloro-2,5-dimethyl-3-heptanone
E) 3-methyl-4-chloro-1-isopropylpentanone

A) 2-chloro-3-ethyl-1-isopropylbutanone
B) isopropyl-chloromethylbutyl ketone
C) 2-butylchloroisobutanoyl methane
D) 4-chloro-2,5-dimethyl-3-heptanone
E) 3-methyl-4-chloro-1-isopropylpentanone

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
54
Formic acid is the common name for pentanoic acid.

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
55
Name the following compound.
CH3COOH
CH3COOH

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
56
Name the following compound. 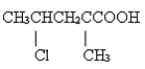
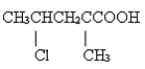

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
57
The name methanal is the systematic name for
A) acetone
B) formaldehyde
C) rubbing alcohol
D) acetaldehyde
E) water
A) acetone
B) formaldehyde
C) rubbing alcohol
D) acetaldehyde
E) water

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
58
Teflon is an example of a
A) copolymer
B) homopolymer
C) dimer
D) two of these
E) none of these
A) copolymer
B) homopolymer
C) dimer
D) two of these
E) none of these

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck



