Deck 12: Chemical Bonding
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/110
Play
Full screen (f)
Deck 12: Chemical Bonding
1
Would CO2 be classified as ionic or covalent?
covalent
2
Would MgCl2 be classified as ionic or covalent?
ionic
3
Would CoCl2 be classified as ionic or covalent?
ionic
4
In general, a larger atom has a smaller electronegativity.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
5
Would K2O be classified as ionic or covalent?

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
6
Covalent bonding occurs when a metal reacts with a nonmetal.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
7
Which of the following bonds would be the most polar without being considered ionic?
A) Mg-O
B) C-O
C) O-O
D) Si-O
E) N-O
A) Mg-O
B) C-O
C) O-O
D) Si-O
E) N-O

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
8
Which of the following bonds would be the least polar yet still be considered polar covalent?
A) Mg-O
B) C-O
C) O-O
D) Si-O
E) N-O
A) Mg-O
B) C-O
C) O-O
D) Si-O
E) N-O

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
9
A bond is a force that holds groups of two or more atoms together and makes them function as a unit.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
10
The greater the difference in electronegativity between two bonded atoms, the more polar the bond.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
11
CH4 has ionic bonds.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
12
Covalent bonding occurs when electrons are shared by nuclei.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
13
Consider the drawings below: 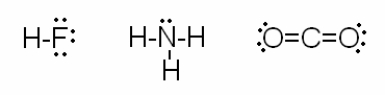 Which of the following statements are true? I. The electrons in each molecule tend to be attracted to the most electronegative element.
Which of the following statements are true? I. The electrons in each molecule tend to be attracted to the most electronegative element.
II) Each molecular drawing follows the localized electron model.
III) Both HF and CO2 are linear molecules and therefore nonpolar.
IV) The bond angles of NH3 are slightly less than 109.5o because the lone pair compresses the angles between the bonding pairs.
A) I, III, IV
B) I, II, IV
C) I, II, III
D) II, IV
E) All of the above statements (I - IV) are correct.
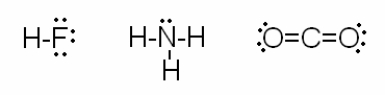 Which of the following statements are true? I. The electrons in each molecule tend to be attracted to the most electronegative element.
Which of the following statements are true? I. The electrons in each molecule tend to be attracted to the most electronegative element.II) Each molecular drawing follows the localized electron model.
III) Both HF and CO2 are linear molecules and therefore nonpolar.
IV) The bond angles of NH3 are slightly less than 109.5o because the lone pair compresses the angles between the bonding pairs.
A) I, III, IV
B) I, II, IV
C) I, II, III
D) II, IV
E) All of the above statements (I - IV) are correct.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
14
Would OCl2 be classified as ionic or covalent?

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
15
Would NaBr be classified as ionic or covalent?

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
16
N2 is an example of a covalent bond.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
17
Rank the following bonds from least polar to most polar: Si-Cl P-Cl Mg-Cl S-Cl
A) S-Cl, P-Cl, Mg-Cl, Si-Cl
B) P-Cl, S-Cl, Si-Cl, Mg-Cl
C) Mg-Cl, Si-Cl, P-Cl, S-Cl
D) Mg-Cl, S-Cl, P-Cl, Si-Cl
E) S-Cl, P-Cl, Si-Cl, Mg-Cl
A) S-Cl, P-Cl, Mg-Cl, Si-Cl
B) P-Cl, S-Cl, Si-Cl, Mg-Cl
C) Mg-Cl, Si-Cl, P-Cl, S-Cl
D) Mg-Cl, S-Cl, P-Cl, Si-Cl
E) S-Cl, P-Cl, Si-Cl, Mg-Cl

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
18
Would NH3 be classified as ionic or covalent?

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
19
Would CH4 be classified as ionic or covalent?

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
20
Ionic bonding occurs between atoms with small differences in electronegativities.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
21
Which if the following compounds contains one or more covalent bonds?
A) CS2
B) LiCl
C) Na2O
D) CaCl2
E) MgS
A) CS2
B) LiCl
C) Na2O
D) CaCl2
E) MgS

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
22
Is the molecule S8 nonpolar or polar?

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
23
Draw the Lewis structures for the following compounds to assist you in answering this question. CBr2H2 BH3 XeCl4 SF4 HCl
Which compound has a see-saw shape?
A) CBr2H2
B) BH3
C) XeCl4
D) SF4
E) HCl
Which compound has a see-saw shape?
A) CBr2H2
B) BH3
C) XeCl4
D) SF4
E) HCl

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
24
The most electronegative element of those listed is
A) B
B) Ga
C) Tl
D) Al
E) In
A) B
B) Ga
C) Tl
D) Al
E) In

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
25
Would AlCl3 be classified as ionic or covalent?

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
26
Order the following bonds from the least polar to the most polar. N-O, Ca-O, C-O, O-O, Ni-O
A) O-O < N-O < C-O < Ca-O < Ni-O
B) O-O < C-O < N-O < Ni-O < Ca-O
C) Ca-O < Ni-O < C-O < N-O < O-O
D) O-O < N-O < C-O < Ni-O < Ca-O
E) Ni-O < Ca-O < C-O < N-O < O-O
A) O-O < N-O < C-O < Ca-O < Ni-O
B) O-O < C-O < N-O < Ni-O < Ca-O
C) Ca-O < Ni-O < C-O < N-O < O-O
D) O-O < N-O < C-O < Ni-O < Ca-O
E) Ni-O < Ca-O < C-O < N-O < O-O

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
27
Which of the following elements has the lowest electronegativity?
A) Cs
B) Na
C) Be
D) S
E) Br
A) Cs
B) Na
C) Be
D) S
E) Br

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
28
Is the molecule CF4 nonpolar or polar?

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
29
Draw the Lewis structures for the following compounds to assist you in answering this question. CBr2H2 BH3 XeCl4 SF4 HCl
Which compound has bond angles of 109.5° around the central atom?
A) CBr2H2
B) BH3
C) XeCl4
D) SF4
E) HCl
Which compound has bond angles of 109.5° around the central atom?
A) CBr2H2
B) BH3
C) XeCl4
D) SF4
E) HCl

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
30
Which of the following contains only nonpolar bonds?
A) CH4
B) HCl
C) H2O
D) Mg3N2
E) Cl2
A) CH4
B) HCl
C) H2O
D) Mg3N2
E) Cl2

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
31
Draw the Lewis structures for the following compounds to assist you in answering this question. CBr2H2 BH3 XeCl4 SF4 HCl
How many of the compounds are nonpolar?
A) 1
B) 2
C) 3
D) 4
E) 5
How many of the compounds are nonpolar?
A) 1
B) 2
C) 3
D) 4
E) 5

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
32
Which of the following has only nonpolar covalent bonds?
A) N2
B) CO
C) HI
D) CCl4
E) NaCl
A) N2
B) CO
C) HI
D) CCl4
E) NaCl

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
33
Which of the following compounds contains an ionic bond?
A) HCl (g)
B) NaCl
C) CCl4
D) SO2
E) O2
A) HCl (g)
B) NaCl
C) CCl4
D) SO2
E) O2

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
34
Would SF4 be classified as ionic or covalent?

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
35
Which of the following has nonpolar bonds?
A) I2
B) H2S
C) OF2
D) HF
E) All are nonpolar.
A) I2
B) H2S
C) OF2
D) HF
E) All are nonpolar.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
36
The most electronegative element of those listed is
A) Zn
B) Si
C) Sr
D) Ba
E) Zr
A) Zn
B) Si
C) Sr
D) Ba
E) Zr

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
37
Is the molecule H2S nonpolar or polar?

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
38
Which of the following elements has the lowest electronegativity?
A) Sr
B) P
C) Mn
D) N
E) H
A) Sr
B) P
C) Mn
D) N
E) H

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
39
Which of these elements has the highest electronegativity?
A) Y
B) I
C) Sb
D) Sr
E) In
A) Y
B) I
C) Sb
D) Sr
E) In

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
40
The least electronegative element of those listed is
A) O
B) Pb
C) Ba
D) Cu
E) Se
A) O
B) Pb
C) Ba
D) Cu
E) Se

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
41
Which of the following has the smallest radius?
A) S2-
B) Cl-
C) Ar
D) K+
E) Ca2+
A) S2-
B) Cl-
C) Ar
D) K+
E) Ca2+

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
42
The electron configuration for Ca2+ is identical to that of
A) Ne
B) Kr
C) Ca
D) Ar
A) Ne
B) Kr
C) Ca
D) Ar

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
43
The electron configuration for the bromide ion is identical to that of
A) Br
B) Kr
C) K
D) I-
E) none of these
A) Br
B) Kr
C) K
D) I-
E) none of these

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
44
One of the most important characteristics of the water molecule is its ______________, which allows it to surround and attract both positive and negative ions.
A) polarity
B) strength
C) magnetism
D) fluidity
E) stability
A) polarity
B) strength
C) magnetism
D) fluidity
E) stability

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
45
A nitrogen atom needs to gain _____ electrons to achieve a noble gas configuration.
A) 3
B) 2
C) 4
D) 5
E) 1
A) 3
B) 2
C) 4
D) 5
E) 1

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
46
In ionic bonding
A) the electrons are shared between the atoms.
B) the process of forming an ionic bond is highly endothermic overall.
C) the bonding that occurs is usually between two nonmetal atoms.
D) a noble gas configuration is formed for each element or ion.
E) At least two of the above statements are correct.
A) the electrons are shared between the atoms.
B) the process of forming an ionic bond is highly endothermic overall.
C) the bonding that occurs is usually between two nonmetal atoms.
D) a noble gas configuration is formed for each element or ion.
E) At least two of the above statements are correct.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
47
Which of the following atoms has the greatest electronegativity?
A) Na
B) Rb
C) Cl
D) Se
A) Na
B) Rb
C) Cl
D) Se

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
48
When a molecule has a center of positive charge and a center of negative charge, it is said to have a ______________.
A) magnetic attraction
B) diatomic bond
C) double bond
D) polyatomic ion
E) dipole moment
A) magnetic attraction
B) diatomic bond
C) double bond
D) polyatomic ion
E) dipole moment

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
49
Which of the following species would be expected to have the lowest ionization energy?
A) Br-
B) Kr
C) Se2-
D) Sr2+
E) Rb+
A) Br-
B) Kr
C) Se2-
D) Sr2+
E) Rb+

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
50
Which element or ion listed below has the electron configuration 1s22s22p6?
A) Na+
B) Al3+
C) F-
D) Ne
E) all of these
A) Na+
B) Al3+
C) F-
D) Ne
E) all of these

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
51
Which of the following has primarily ionic bonding?
A) N2O3
B) Na2O
C) CO2
D) CCl4
E) none of these
A) N2O3
B) Na2O
C) CO2
D) CCl4
E) none of these

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
52
Which element or ion listed below has the electron configuration 1s22s22p63s23p6?
A) Cl
B) Br-
C) Se
D) Ca2+
E) two of these
A) Cl
B) Br-
C) Se
D) Ca2+
E) two of these

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
53
Which of the following bonds does not have a dipole moment?
A) O-O
B) B-H
C) N-O
D) O-H
E) S-H
A) O-O
B) B-H
C) N-O
D) O-H
E) S-H

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
54
The number of polar covalent bonds in OF2 is
A) 2
B) 1
C) 3
D) 4
E) none of these
A) 2
B) 1
C) 3
D) 4
E) none of these

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
55
Which of the following has the largest radius?
A) S2-
B) Cl-
C) Ar
D) K+
E) Ca2+
A) S2-
B) Cl-
C) Ar
D) K+
E) Ca2+

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
56
Arrange the following elements in order of increasing electronegativity (from smallest to largest).
A) Li < C < N < F
B) C < N < F < Li
C) N < C < Li < F
D) C < F < Li < N
E) F < N < C < Li
A) Li < C < N < F
B) C < N < F < Li
C) N < C < Li < F
D) C < F < Li < N
E) F < N < C < Li

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
57
Which of the following ions has the same electron configuration as an argon atom?
A) Br-
B) S3-
C) P3+
D) K+
E) Ca+
A) Br-
B) S3-
C) P3+
D) K+
E) Ca+

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
58
If atom X forms a diatomic molecule with itself, the bond is
A) ionic
B) polar covalent
C) nonpolar covalent
D) polar coordinate covalent
E) none of these
A) ionic
B) polar covalent
C) nonpolar covalent
D) polar coordinate covalent
E) none of these

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
59
The F- and O2- ions have the same electron configuration.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
60
The electron configuration 1s22s22p63s23p64s23d104p6 is the correct electron configuration for the most stable form of which ion?
A) the strontium ion
B) the calcium ion
C) the krypton ion
D) the barium ion
E) the xenon ion
A) the strontium ion
B) the calcium ion
C) the krypton ion
D) the barium ion
E) the xenon ion

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
61
Draw the Lewis electron structure for the silicon atom.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
62
Draw the Lewis electron structure for the sulfide ion.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
63
The formula of the compound formed in the reaction between potassium and oxygen is
A) K2O
B) KO2
C) K2O3
D) KO
E) none of these
A) K2O
B) KO2
C) K2O3
D) KO
E) none of these

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
64
Which element or ion listed below has the electron configuration 1s22s22p63s23p6?
A) Ca+
B) Na+
C) K+
D) Cl
E) none of these
A) Ca+
B) Na+
C) K+
D) Cl
E) none of these

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
65
Which element or ion listed below has the electron configuration 1s22s22p63s23p6?
A) S
B) Ne
C) Cl
D) S2-
E) none of these
A) S
B) Ne
C) Cl
D) S2-
E) none of these

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
66
Write the electron configuration for Sr2+.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
67
Which one of the following species has the same electron configuration as an atom of argon?
A) S-
B) Cl2-
C) K
D) Ca2+
E) Kr
A) S-
B) Cl2-
C) K
D) Ca2+
E) Kr

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
68
Magnesium reacts with bromine to form
A) MgBr2
B) MgBr
C) Mg2Br
D) Mg2Br3
E) none of these
A) MgBr2
B) MgBr
C) Mg2Br
D) Mg2Br3
E) none of these

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
69
Write the electron configuration for Br-.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
70
Which of the following compounds is the product of the reaction Mg + O2?
A) MgO
B) MgO2
C) MgO3
D) Mg2O
E) Mg2O3
A) MgO
B) MgO2
C) MgO3
D) Mg2O
E) Mg2O3

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
71
Write the electron configuration for Cl-.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
72
When they react chemically, the alkali metals (Group 1A)
A) gain 1 electron
B) gain 7 electrons
C) gain or lose 7 electrons
D) lose 1 electron
A) gain 1 electron
B) gain 7 electrons
C) gain or lose 7 electrons
D) lose 1 electron

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
73
Write the electron configuration for Al3+.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
74
Which element listed below has the electron configuration 1s22s22p63s23p2 ?
A) Si
B) P
C) Ge
D) C
E) none of these
A) Si
B) P
C) Ge
D) C
E) none of these

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
75
Magnesium reacts with oxygen to form
A) MgO
B) MgO2
C) Mg2O
D) Mg2O3
E) none of these
A) MgO
B) MgO2
C) Mg2O
D) Mg2O3
E) none of these

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
76
Which of the following is the product of the reaction Al + O2?
A) AlO
B) AlO2
C) AlO3
D) Al3O2
E) Al2O3
A) AlO
B) AlO2
C) AlO3
D) Al3O2
E) Al2O3

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
77
Complete the table by giving the predicted formulas of the compounds formed between the elements listed.
Br
S
Na
______________
______________
Mg
______________
______________
Al
______________
______________
Br
S
Na
______________
______________
Mg
______________
______________
Al
______________
______________

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
78
How many lone pairs of electrons are in the Lewis structure for compound, CH4?
A) 0
B) 1
C) 2
D) 3
E) none of these
A) 0
B) 1
C) 2
D) 3
E) none of these

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
79
Draw the Lewis electron structure for the chlorine atom.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
80
Draw the Lewis electron structure for the sulfur atom.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck



