Deck 15: Chirality: the Handedness of Molecules
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/92
Play
Full screen (f)
Deck 15: Chirality: the Handedness of Molecules
1
Which of the following three isomeric alcohols, 2-pentanol, 2-methyl-2-butanol, and 3-methyl-2-butanol, are chiral?
A) only 2-pentanol
B) only 2-methyl-2-butanol
C) both 2-pentanol and 2-methyl-2-butanol
D) both 2-pentanol and 3-methyl-2-butanol
A) only 2-pentanol
B) only 2-methyl-2-butanol
C) both 2-pentanol and 2-methyl-2-butanol
D) both 2-pentanol and 3-methyl-2-butanol
D
2
Given the compounds 2-methyl-1-pentene and 3-methyl-1-pentene, which of the following statements is true?
A) Only 2-methyl-1-pentene is chiral.
B) Only 3-methyl-1-pentene is chiral.
C) Both are chiral.
D) Neither is chiral.
A) Only 2-methyl-1-pentene is chiral.
B) Only 3-methyl-1-pentene is chiral.
C) Both are chiral.
D) Neither is chiral.
B
3
Given the compounds 2,3-dimethylpentane and 2,4-dimethylpentane, which of the following is true?
A) Only 2,3-dimethylpentane is chiral.
B) Only 2,4-dimethylpentane is chiral.
C) Both are chiral.
D) Neither is chiral.
A) Only 2,3-dimethylpentane is chiral.
B) Only 2,4-dimethylpentane is chiral.
C) Both are chiral.
D) Neither is chiral.
A
4
Given the compounds 1,1-diethyl-4-methylcyclohexane, and 4-ethyl-1,1-dimethylcyclohexane, which of the following statements is true?
A) Only 1,1-diethyl-4-methylcyclohexane is chiral.
B) Only 4-ethyl-1,1-dimethylcyclohexane is chiral.
C) Both are chiral.
D) Neither is chiral.
A) Only 1,1-diethyl-4-methylcyclohexane is chiral.
B) Only 4-ethyl-1,1-dimethylcyclohexane is chiral.
C) Both are chiral.
D) Neither is chiral.

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
5
Given the compounds 1,1,3-trimethylcyclohexane, and 1,1,4-trimethylcyclohexane, which of the following statements is true?
A) Only 1,1,3-trimethylcyclohexane is chiral.
B) Only 1,1,4-trimethylcylcohexane is chiral.
C) Both are chiral.
D) Neither is chiral.
A) Only 1,1,3-trimethylcyclohexane is chiral.
B) Only 1,1,4-trimethylcylcohexane is chiral.
C) Both are chiral.
D) Neither is chiral.

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
6
Which of the following three isomeric alcohols, 2,2-dimethyl-1-butanol, 2-methyl-2-pentanol, and 2-methyl-3-pentanol, are chiral?
A) Only 2,2-dimethyl-1-butanol.
B) Only 2-methyl-3-pentanol.
C) Both 2,2-dimethyl-1-butanol and 2-methyl-3-pentanol.
D) Both 2-methyl-2-pentanol and 2-methyl-3-pentanol.
A) Only 2,2-dimethyl-1-butanol.
B) Only 2-methyl-3-pentanol.
C) Both 2,2-dimethyl-1-butanol and 2-methyl-3-pentanol.
D) Both 2-methyl-2-pentanol and 2-methyl-3-pentanol.

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
7
Which of the following would represent a racemic mixture?
A) a 1:1 mixture of 1-butanol and 2-butanol
B)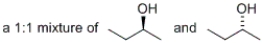
C)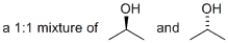
D) all of the above
A) a 1:1 mixture of 1-butanol and 2-butanol
B)

C)

D) all of the above

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
8
Which of the structures given below represents the enantiomer of the following compound: 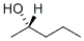 ?
?
A)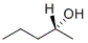 only
only
B)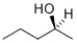 only
only
C) both a and b
D) neither b nor b
 ?
?A)
 only
onlyB)
 only
onlyC) both a and b
D) neither b nor b

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
9
There are six alcohols with the molecular formula C5H11OH. How many of these are chiral?
A) 0
B) 1
C) 2
D) 3
A) 0
B) 1
C) 2
D) 3

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
10
Which of the three isomeric alcohols, 2-butanol, 2-methyl-1-propanol, and 2-methyl-2-propanol, are chiral?
A) only 2-butanol
B) only 2-methyl-1-propanol
C) both 2-butanol and 2-methyl-1-propanol
D) both 2-methyl-1-propanol and 2-methyl-2-propanol
A) only 2-butanol
B) only 2-methyl-1-propanol
C) both 2-butanol and 2-methyl-1-propanol
D) both 2-methyl-1-propanol and 2-methyl-2-propanol

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
11
Which of the following is true of a molecule which contains one stereocenter?
A) It will never be chiral.
B) It will sometimes be chiral.
C) It will always be chiral.
D) There is no relationship between chirality and the presence of a stereocenter.
A) It will never be chiral.
B) It will sometimes be chiral.
C) It will always be chiral.
D) There is no relationship between chirality and the presence of a stereocenter.

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
12
What is the fewest number of carbon atoms present in a chiral noncyclic alkane?
A) 4
B) 5
C) 6
D) 7
A) 4
B) 5
C) 6
D) 7

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
13
Given the compounds 2-methyl-1-pentene and 2-methyl-2-pentene, which of the following statements is true?
A) Only 2-methyl-1-pentene is chiral.
B) Only 2-methyl-2-pentene is chiral.
C) Both are chiral.
D) Neither is chiral.
A) Only 2-methyl-1-pentene is chiral.
B) Only 2-methyl-2-pentene is chiral.
C) Both are chiral.
D) Neither is chiral.

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
14
Which of the following statements is true?
A) Biologically important organic molecules never exhibit enantiomerism.
B) Very few biologically important organic molecules exhibit enantiomerism.
C) The vast majority of biologically important organic molecules exhibit enantiomerism.
D) All biologically important organic molecules exhibit enantiomerism.
A) Biologically important organic molecules never exhibit enantiomerism.
B) Very few biologically important organic molecules exhibit enantiomerism.
C) The vast majority of biologically important organic molecules exhibit enantiomerism.
D) All biologically important organic molecules exhibit enantiomerism.

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
15
How many stereocenters are present in the following compound? 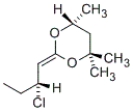
A) 1
B) 2
C) 3
D) 4

A) 1
B) 2
C) 3
D) 4

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
16
Given the compounds 2,3-dimethylpentane and 3-methylhexane, which of the following is true?
A) Only 2,3-dimethylpentane is chiral.
B) Only 3-methylhexane is chiral.
C) Both are chiral.
D) Neither is chiral.
A) Only 2,3-dimethylpentane is chiral.
B) Only 3-methylhexane is chiral.
C) Both are chiral.
D) Neither is chiral.

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
17
Given the compounds 3-methyl-1-pentene and trans-3-methyl-2-pentene, which of the following statements is true?
A) Only 3-methyl-1-pentene is chiral.
B) Only trans-3-methyl-2-pentene is chiral.
C) Both are chiral.
D) Neither is chiral.
A) Only 3-methyl-1-pentene is chiral.
B) Only trans-3-methyl-2-pentene is chiral.
C) Both are chiral.
D) Neither is chiral.

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
18
Given the compounds cis-1,4-dimethylcyclohexane, and trans-1,4-dimethylcyclohexane, which of the following statements is/are true? (i) these two compounds are related as stereoisomers;
(ii) cis-1,4-dimethylcyclohexane is chiral;
(iii) trans-1,4-dimethylcyclohexane is chiral.
A) only (i) is true
B) only (i) and (ii) are true
C) only (ii) and (iii) are true
D) only (i) and (iii) are true
(ii) cis-1,4-dimethylcyclohexane is chiral;
(iii) trans-1,4-dimethylcyclohexane is chiral.
A) only (i) is true
B) only (i) and (ii) are true
C) only (ii) and (iii) are true
D) only (i) and (iii) are true

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
19
If a carbon atom is a stereocenter, which of the following statements must be true about that carbon?
A) It has four single bonds.
B) It has a double bond and two single bonds.
C) It has two double bonds.
D) Any of these bonding arrangements can be associated with a stereocenter.
A) It has four single bonds.
B) It has a double bond and two single bonds.
C) It has two double bonds.
D) Any of these bonding arrangements can be associated with a stereocenter.

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
20
There are four alcohols with the molecular formula C4H9OH. How many of these are chiral?
A) 0
B) 1
C) 2
D) 3
A) 0
B) 1
C) 2
D) 3

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
21
Among the following groups, which is the correct order of priorities in the R,S system? 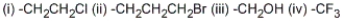
A) (ii) > (i) > (iii) > (iv)
B) (iii) > (iv) > (ii) > (i)
C) (i) > (iii) > (ii) > (iv)
D) (iv) > (iii) > (i) > (ii)
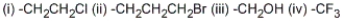
A) (ii) > (i) > (iii) > (iv)
B) (iii) > (iv) > (ii) > (i)
C) (i) > (iii) > (ii) > (iv)
D) (iv) > (iii) > (i) > (ii)

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
22
Which of the following is the correct order of priorities in the R,S system?
A) alkyl > amino > hydroxyl > thiol
B) alkyl > hydroxyl > amino > thiol
C) thiol > amino > hydroxyl > alkyl
D) thiol > hydroxyl > amino > alkyl
A) alkyl > amino > hydroxyl > thiol
B) alkyl > hydroxyl > amino > thiol
C) thiol > amino > hydroxyl > alkyl
D) thiol > hydroxyl > amino > alkyl

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
23
Which of the following molecules has an R configuration?
A)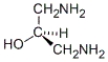
B)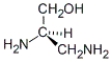
C) both a and b
D) neither a nor b
A)

B)

C) both a and b
D) neither a nor b

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
24
Among the following groups, what is the correct priority ordering in the R,S system? 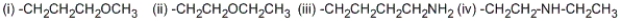
A) (i) > (ii) > (iii) > (iv)
B) (ii) > (i) > (iv) > (iii)
C) (iv) > (ii) > (i) > (iii)
D) (ii) > (iv) > (i) > (iii)
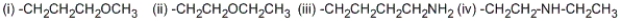
A) (i) > (ii) > (iii) > (iv)
B) (ii) > (i) > (iv) > (iii)
C) (iv) > (ii) > (i) > (iii)
D) (ii) > (iv) > (i) > (iii)

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
25
In the R,S system, which of the following groups has lowest priority?
A) alkyl
B) amino
C) hydroxyl
D) thiol
A) alkyl
B) amino
C) hydroxyl
D) thiol

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
26
Which of the following molecules has an R configuration?
A)
B)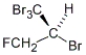
C) both a and b
D) neither a nor b
A)

B)

C) both a and b
D) neither a nor b

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
27
In the R,S system, which of the following groups has lowest priority?
A) aldehyde
B) carboxyl
C) ketone
D) primary alcohol
A) aldehyde
B) carboxyl
C) ketone
D) primary alcohol

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
28
Which of the following is the correct order of priorities in the R,S system?
A) aldehyde > carboxyl > ketone > primary alcohol
B) carboxyl > ketone > aldehyde > primary alcohol
C) primary alcohol > aldehyde > ketone > carboxyl
D) primary alcohol > ketone > carboxyl > aldehyde
A) aldehyde > carboxyl > ketone > primary alcohol
B) carboxyl > ketone > aldehyde > primary alcohol
C) primary alcohol > aldehyde > ketone > carboxyl
D) primary alcohol > ketone > carboxyl > aldehyde

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
29
What are the R/S configurations at the following stereocenters? 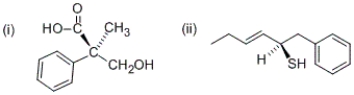
A) (i) R (ii) R
B) (i) R (ii) S
C) (i) S (ii) R
D) (i) S (ii) S

A) (i) R (ii) R
B) (i) R (ii) S
C) (i) S (ii) R
D) (i) S (ii) S

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
30
In the R,S system, which of the following alkyl groups has lowest priority?
A) butyl
B) ethyl
C) methyl
D) propyl
A) butyl
B) ethyl
C) methyl
D) propyl

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
31
Which of the following compounds can exist as a pair of enantiomers? 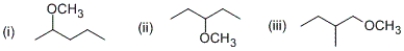
A) (i) and (ii) only
B) (ii) and (iii) only
C) (i) and (iii) only
D) (i) only

A) (i) and (ii) only
B) (ii) and (iii) only
C) (i) and (iii) only
D) (i) only

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
32
In the R,S system, which of the following alkyl groups has highest priority?
A) butyl
B) ethyl
C) methyl
D) propyl
A) butyl
B) ethyl
C) methyl
D) propyl

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
33
In the R,S system, which of the following groups has highest priority?
A) alkyl
B) amino
C) hydroxyl
D) thiol
A) alkyl
B) amino
C) hydroxyl
D) thiol

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
34
In the R,S system, which of the following groups has highest priority?
A) aldehyde
B) carboxyl
C) ketone
D) primary alcohol
A) aldehyde
B) carboxyl
C) ketone
D) primary alcohol

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
35
Which of the following molecules has an R configuration?
A)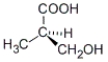
B)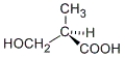
C) both a and b
D) neither a nor b
A)

B)

C) both a and b
D) neither a nor b

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
36
Which of the following molecules has an S configuration?
A)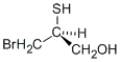
B)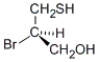
C) both a and b
D) neither a nor b
A)

B)

C) both a and b
D) neither a nor b

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
37
Which of the following is the correct order of priorities in the R,S system?
A) butyl < ethyl < methyl < propyl
B) propyl < methyl < ethyl < butyl
C) butyl < propyl < ethyl < methyl
D) methyl < ethyl < propyl < butyl
A) butyl < ethyl < methyl < propyl
B) propyl < methyl < ethyl < butyl
C) butyl < propyl < ethyl < methyl
D) methyl < ethyl < propyl < butyl

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
38
What are the R/S configurations at the following stereocenters? 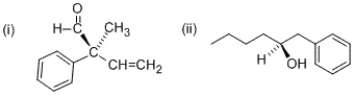
A) (i) R (ii) R
B) (i) R (ii) S
C) (i) S (ii) R
D) (i) S (ii) S

A) (i) R (ii) R
B) (i) R (ii) S
C) (i) S (ii) R
D) (i) S (ii) S

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
39
Which of the following molecules has an R configuration?
A)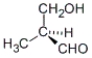
B)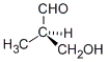
C) both a and b
D) neither a nor b
A)

B)

C) both a and b
D) neither a nor b

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
40
Which of the following is the correct order of priorities in the R,S system?
A) tert-butyl > butyl > isopropyl > propyl
B) tert-butyl > isopropyl > butyl > propyl
C) isopropyl > tert-butyl > propyl > butyl
D) propyl > butyl >isopropyl > tert-butyl
A) tert-butyl > butyl > isopropyl > propyl
B) tert-butyl > isopropyl > butyl > propyl
C) isopropyl > tert-butyl > propyl > butyl
D) propyl > butyl >isopropyl > tert-butyl

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
41
How many stereocenters are present in the following carbohydrate? 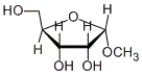
A) 1
B) 2
C) 3
D) 4

A) 1
B) 2
C) 3
D) 4

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
42
Given the three molecules 1-methylcyclohexanol, trans-2-methylcyclohexanol, and trans-4-methylcyclohexanol, which of the following statements is true?
A) All three molecules are chiral.
B) Only 1-methylcyclohexanol and trans-2-methylcyclohexanol are chiral.
C) Only trans-2-methylcyclohexanol is chiral.
D) Only trans-2-methylcyclohexanol and trans-4-methylcyclohexanol are chiral.
A) All three molecules are chiral.
B) Only 1-methylcyclohexanol and trans-2-methylcyclohexanol are chiral.
C) Only trans-2-methylcyclohexanol is chiral.
D) Only trans-2-methylcyclohexanol and trans-4-methylcyclohexanol are chiral.

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
43
Which of the following molecules does not have an S configuration at a stereocenter?
A)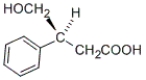
B)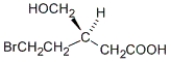
C)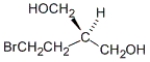
D) they all have an (S)-stereocenter
A)

B)

C)

D) they all have an (S)-stereocenter

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
44
How many stereoisomers are possible for 3-ethyl-4-methyl-2-hexanol?
A) 2
B) 4
C) 8
D) 16
A) 2
B) 4
C) 8
D) 16

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
45
Which of the following is a representation of the enantiomer of the following substance? 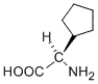
A)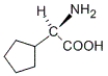
B)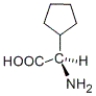
C) both a and b
D) neither a nor b

A)

B)

C) both a and b
D) neither a nor b

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
46
How many stereoisomers are possible for the following compound? 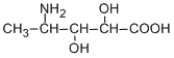
A) 2
B) 4
C) 8
D) 16

A) 2
B) 4
C) 8
D) 16

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
47
Illustrated here are the four stereoisomers of an amino acid which has two stereocenters. Which structure(s) is/are diastereomer(s) of structure A? 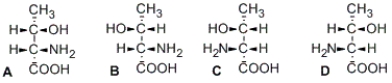
A) B only
B) C only
C) D only
D) B and D only

A) B only
B) C only
C) D only
D) B and D only

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
48
Which of the following pairs of stereoisomers are related as enantiomers? 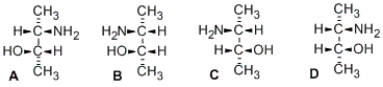
A) A and B; C and D
B) A and C; B and D
C) A and D; B and C
D) none of them

A) A and B; C and D
B) A and C; B and D
C) A and D; B and C
D) none of them

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
49
Progesterone, which is present in humans as a single stereoisomer, has six stereocenters. Human progesterone is one of how many possible stereoisomers for this structure?
A) 6
B) 32
C) 64
D) 128
A) 6
B) 32
C) 64
D) 128

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
50
How many stereocenters are present in 3-methyl-2-pentanol?
A) 1
B) 2
C) 3
D) 5
A) 1
B) 2
C) 3
D) 5

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
51
Illustrated here are the four stereoisomers of an amino acid which has two stereocenters. Which structure(s) is/are the enantiomer(s) of structure A? 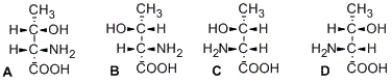
A) B only
B) C only
C) D only
D) C and D only

A) B only
B) C only
C) D only
D) C and D only

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
52
How many stereoisomers are possible for the following triglyceride? (Include all types of stereoisomerism!) 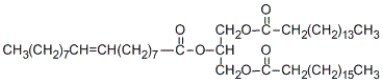
A) 1
B) 2
C) 4
D) 8

A) 1
B) 2
C) 4
D) 8

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
53
How many stereocenters are present in the following triglyceride? 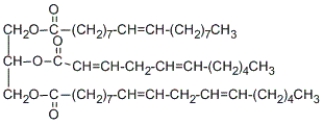
A) 0
B) 1
C) 4
D) 6
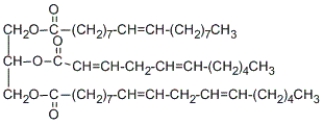
A) 0
B) 1
C) 4
D) 6

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
54
How many chiral stereoisomers are possible for 2-chlorocyclopentanol?
A) 1
B) 2
C) 3
D) 4
A) 1
B) 2
C) 3
D) 4

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
55
How many stereoisomers are possible for the following triglyceride? (Hint: include all types of stereoisomers!) 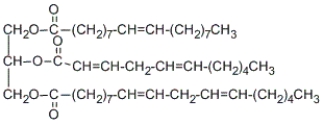
A) 2
B) 8
C) 32
D) 64
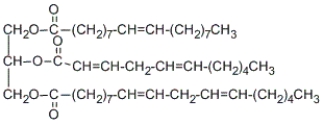
A) 2
B) 8
C) 32
D) 64

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
56
What is the maximum number of stereoisomers possible for a molecule which has three stereocenters (assuming no other structural features that lead to steroisomerism)?
A) 2
B) 4
C) 8
D) 16
A) 2
B) 4
C) 8
D) 16

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
57
Which of the following is a representation of the enantiomer of the following substance? 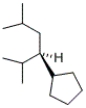
A)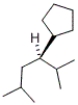
B)
C) both a and b
D) neither a nor b

A)

B)

C) both a and b
D) neither a nor b

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
58
The sugar in deoxyribonucleic acid, DNA, is called 2-deoxy-D-ribose. It is found as a single stereoisomer. How many enantiomers of 2-deoxy-D-ribose exist?
A) 0
B) 1
C) 2
D) 4
A) 0
B) 1
C) 2
D) 4

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
59
How many stereoisomers exist for the following compound? ? 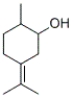
A) 1
B) 2
C) 4
D) 8

A) 1
B) 2
C) 4
D) 8

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
60
How many stereocenters are present in 2,4-dichloro-3-hexanol?
A) 1
B) 2
C) 3
D) 5
A) 1
B) 2
C) 3
D) 5

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
61
In its pyranose form, naturally occurring D-glucose has five stereocenters. How many possible stereoisomers exist for this structure?
A) 5
B) 10
C) 25
D) 32
A) 5
B) 10
C) 25
D) 32

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
62
Chymotrypsin has 251 stereocenters. What is the maximum number of stereoisomers possible for a molecule with this number of stereocenters?
A) 251
B) 2 × 251
C) 2251
D) 10251
A) 251
B) 2 × 251
C) 2251
D) 10251

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
63
If the specific rotation of the (R)-enantiomer of a compound is -4.8o, what will the specific rotation be for the (S)-enantiomer of the compound?
A) +4.8°
B) -4.8°
C) 0°
D) There is no way to tell.
A) +4.8°
B) -4.8°
C) 0°
D) There is no way to tell.

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
64
Which of the following is true of the ibuprofen-containing drugs Advil and Motrin?
A) They are both sold as racemic mixtures.
B) For both only a single enantiomer is manufactured and sold.
C) Advil is sold as a racemic mixture but Motrin is sold as a single enantiomer.
D) Motrin is sold as a racemic mixture but Advil is sold as a single enantiomer.
A) They are both sold as racemic mixtures.
B) For both only a single enantiomer is manufactured and sold.
C) Advil is sold as a racemic mixture but Motrin is sold as a single enantiomer.
D) Motrin is sold as a racemic mixture but Advil is sold as a single enantiomer.

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
65
Which of the following is true of chiral drugs that are administered as single enantiomers?
A) They all have the R configuration.
B) They all have the S configuration.
C) Their enantiomers are always highly toxic.
D) None of the above is correct.
A) They all have the R configuration.
B) They all have the S configuration.
C) Their enantiomers are always highly toxic.
D) None of the above is correct.

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
66
The actual rotation of a sample measured in a 10 cm cell can be directly reported as the specific rotation if the concentration of the sample is which of the following?
A) 1 M
B) 1 mg/L
C) 1 g/mL
D) 1 g/L
A) 1 M
B) 1 mg/L
C) 1 g/mL
D) 1 g/L

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
67
In which of the following are both compounds effective pain relievers?
A) (R)-ibuprofen and (R)-naproxen
B) (S)-ibuprofen and (S)-naproxen
C) (R)-ibuprofen and (S)-naproxen
D) (S)-ibuprofen and (R)-naproxen
A) (R)-ibuprofen and (R)-naproxen
B) (S)-ibuprofen and (S)-naproxen
C) (R)-ibuprofen and (S)-naproxen
D) (S)-ibuprofen and (R)-naproxen

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
68
Which of the following is essentially biologically inactive?
A) (R)-ibuprofen
B) (S)-ibuprofen
C) (R)-naproxen
D) (S)-naproxen
A) (R)-ibuprofen
B) (S)-ibuprofen
C) (R)-naproxen
D) (S)-naproxen

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
69
Which of the following is true of the drugs Motrin (ibuprofen) and Captopril?
A) They are both sold as racemic mixtures.
B) For both only a single enantiomer is manufactured and sold.
C) Motrin is sold as a racemic mixture but Captopril is sold as a single enantiomer.
D) Captopril is sold as a racemic mixture but Motrin is sold as a single enantiomer.
A) They are both sold as racemic mixtures.
B) For both only a single enantiomer is manufactured and sold.
C) Motrin is sold as a racemic mixture but Captopril is sold as a single enantiomer.
D) Captopril is sold as a racemic mixture but Motrin is sold as a single enantiomer.

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
70
When the sample chamber of a polarimeter is empty which of the following statements is true?
A) The maximum transmission of light occurs when the polarizer and analyzer are parallel to each other.
B) The maximum transmission of light occurs when the polarizer and analyzer are perpendicular to each other.
C) The maximum transmission of light occurs when the polarizer and analyzer are at an angle of 120° to each other.
D) Since the instrument is empty the positions of the polarizer and analyzer have no effect on the amount of transmission.
A) The maximum transmission of light occurs when the polarizer and analyzer are parallel to each other.
B) The maximum transmission of light occurs when the polarizer and analyzer are perpendicular to each other.
C) The maximum transmission of light occurs when the polarizer and analyzer are at an angle of 120° to each other.
D) Since the instrument is empty the positions of the polarizer and analyzer have no effect on the amount of transmission.

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
71
The specific rotation of a compound is defined as the observed rotation of the compound when its concentration is 1 g/mL in a sample tube which is 10 cm long. If a certain compound is observed to have a specific rotation of +8.0°, what rotation will be observed if the sample concentration is 0.25 g/mL and the sample tube is 20 cm long?
A) +2°
B) +4°
C) +16°
D) +32°
A) +2°
B) +4°
C) +16°
D) +32°

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
72
Which of the following statements is true of dextrorotatory compounds?
A) They all rotate plane polarized light in a clockwise direction.
B) They all rotate plane polarized light in a counterclockwise direction.
C) They all rotate unpolarized light in a clockwise direction.
D) They all rotate unpolarized light in a counterclockwise direction.
A) They all rotate plane polarized light in a clockwise direction.
B) They all rotate plane polarized light in a counterclockwise direction.
C) They all rotate unpolarized light in a clockwise direction.
D) They all rotate unpolarized light in a counterclockwise direction.

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
73
Which of the following correctly describes the relationship between the R,S system and the actual rotation associated with optically active compounds?
A) All R enantiomers are dextrorotatory.
B) All S enantiomers are dextrorotatory.
C) No S enantiomers are dextrorotatory, but some R enantiomers are levorotatory.
D) There is no general relationship between the R,S designation and the actual direction of rotation of plane polarized light.
A) All R enantiomers are dextrorotatory.
B) All S enantiomers are dextrorotatory.
C) No S enantiomers are dextrorotatory, but some R enantiomers are levorotatory.
D) There is no general relationship between the R,S designation and the actual direction of rotation of plane polarized light.

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
74
How are the following two carbohydrates related to each other? 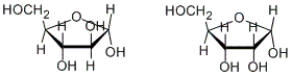
A) enantiomers
B) diastereomers
C) constitutional isomers
D) They are not related to each other.

A) enantiomers
B) diastereomers
C) constitutional isomers
D) They are not related to each other.

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
75
Which of the following correctly describes the relationship between the R,S system and the +/− system associated with optically active compounds?
A) All R enantiomers are (+).
B) All S enantiomers are (+).
C) No S enantiomers are (+), but some R enantiomers are (−).
D) There is no general relationship between the R,S designations and the +/− designations.
A) All R enantiomers are (+).
B) All S enantiomers are (+).
C) No S enantiomers are (+), but some R enantiomers are (−).
D) There is no general relationship between the R,S designations and the +/− designations.

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
76
Which of the following statements is true of levorotatory compounds?
A) They all rotate plane polarized light in a clockwise direction.
B) They all rotate plane polarized light in a counterclockwise direction.
C) They all rotate unpolarized light in a clockwise direction.
D) They all rotate unpolarized light in a counterclockwise direction.
A) They all rotate plane polarized light in a clockwise direction.
B) They all rotate plane polarized light in a counterclockwise direction.
C) They all rotate unpolarized light in a clockwise direction.
D) They all rotate unpolarized light in a counterclockwise direction.

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
77
Lactic acid, shown below, is produced by muscle exercise and can also be found in sour milk. Which of the following statements is true? 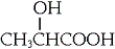
A) The racemic lactic acid produced by muscle exercise is identical to that found in sour milk.
B) The (−)-enantiomer is produced by muscle exercise and is also found in sour milk.
C) The (+)-enantiomer is produced by muscle exercise and is also found in sour milk.
D) Lactic acid produced by muscle exercise is the (+)-enantiomer, but that found in sour milk is the (−)-enantiomer.
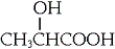
A) The racemic lactic acid produced by muscle exercise is identical to that found in sour milk.
B) The (−)-enantiomer is produced by muscle exercise and is also found in sour milk.
C) The (+)-enantiomer is produced by muscle exercise and is also found in sour milk.
D) Lactic acid produced by muscle exercise is the (+)-enantiomer, but that found in sour milk is the (−)-enantiomer.

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
78
Which of the following correctly describes the relationship between the dextrorotatory/levorotatory system and the +/− system associated with optically active compounds?
A) All dextrorotatory compounds are (+).
B) All levorotatory compounds are (+).
C) No levorotatory compounds are +, but some dextrorotatory compounds are (−).
D) There is no general relationship between the dextrorotatory/levorotatory designations and the +/− designations.
A) All dextrorotatory compounds are (+).
B) All levorotatory compounds are (+).
C) No levorotatory compounds are +, but some dextrorotatory compounds are (−).
D) There is no general relationship between the dextrorotatory/levorotatory designations and the +/− designations.

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
79
If the specific rotation of the (R)-enantiomer of a compound is -4.8o, what will the specific rotation be for a racemic mixture of the compound?
A) +4.8o
B) -4.8o
C) 00
D) There is no way to tell.
A) +4.8o
B) -4.8o
C) 00
D) There is no way to tell.

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
80
In order to distinguish the R and S forms of a chiral molecule with a single stereocenter, an enzyme must have binding sites for how many of the groups on the stereocenter of the molecule?
A) 1
B) 2
C) 3
D) 4
A) 1
B) 2
C) 3
D) 4

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck



