Deck 9: Nuclear Chemistry
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/155
Play
Full screen (f)
Deck 9: Nuclear Chemistry
1
Which of the following Greek letters is not commonly applied to a form of radioactivity?
A) α
B)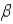
C)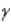
D)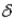
A) α
B)
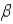
C)
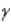
D)
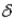
D
2
Which of the following forms of radiation is the least penetrating?
A) α
B)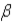
C)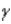
D) positron
A) α
B)
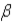
C)
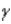
D) positron
A
3
Which of the following is the speed of light?
A) 3.0 × 108 cm/sec
B) 3.0 × 108 m/sec
C) 3.0 × 108 mi/sec
D) 3.0 × 108 mi/hour
A) 3.0 × 108 cm/sec
B) 3.0 × 108 m/sec
C) 3.0 × 108 mi/sec
D) 3.0 × 108 mi/hour
B
4
Which of the following forms of radiation consists of negatively charged particles?
A) α
B)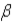
C)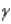
D) none of these
A) α
B)
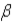
C)
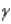
D) none of these

Unlock Deck
Unlock for access to all 155 flashcards in this deck.
Unlock Deck
k this deck
5
Which of the following forms of radiation is the most penetrating?
A) α
B)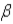
C)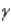
D) positron
A) α
B)
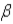
C)
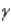
D) positron

Unlock Deck
Unlock for access to all 155 flashcards in this deck.
Unlock Deck
k this deck
6
Which of the following gives the correct order for the energies of portions of the electromagnetic spectrum?
A) radio > infrared > ultraviolet
B) radio > ultraviolet > infrared
C) ultraviolet > infrared > radio
D) ultraviolet > radio > infrared
A) radio > infrared > ultraviolet
B) radio > ultraviolet > infrared
C) ultraviolet > infrared > radio
D) ultraviolet > radio > infrared

Unlock Deck
Unlock for access to all 155 flashcards in this deck.
Unlock Deck
k this deck
7
Which of the following forms of radiation is electrically neutral?
A) α
B)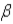
C)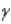
D) none of these
A) α
B)
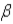
C)
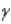
D) none of these

Unlock Deck
Unlock for access to all 155 flashcards in this deck.
Unlock Deck
k this deck
8
Which of the following forms of radiation involves the heaviest particles?
A) α
B)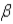
C)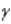
D) positron
A) α
B)
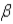
C)
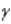
D) positron

Unlock Deck
Unlock for access to all 155 flashcards in this deck.
Unlock Deck
k this deck
9
Which of the following forms of radiation is a form of electromagnetic radiation?
A) α
B)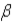
C)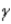
D) all of these
A) α
B)
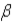
C)
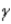
D) all of these

Unlock Deck
Unlock for access to all 155 flashcards in this deck.
Unlock Deck
k this deck
10
Which of the following is the relationship between the wavelength, λ, and frequency, v, of electromagnetic radiation?
A) λ = cν
B) λ = c/ν
C) λ = ν/c
D) λ = 1/cν
A) λ = cν
B) λ = c/ν
C) λ = ν/c
D) λ = 1/cν

Unlock Deck
Unlock for access to all 155 flashcards in this deck.
Unlock Deck
k this deck
11
Which of the following forms of radiation consists of hydrogen nuclei?
A) α
B)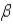
C)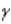
D) none of these
A) α
B)
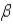
C)
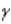
D) none of these

Unlock Deck
Unlock for access to all 155 flashcards in this deck.
Unlock Deck
k this deck
12
Which of the following scientists is not closely associated with early studies of radioactivity?
A) H. Becquerel
B) M. Curie
C) A. Einstein
D) E. Rutherford
A) H. Becquerel
B) M. Curie
C) A. Einstein
D) E. Rutherford

Unlock Deck
Unlock for access to all 155 flashcards in this deck.
Unlock Deck
k this deck
13
Which of the following was Becquerel studying when he accidentally discovered radioactivity?
A) fluorescence
B) phosphorescence
C) photography
D) the photoelectric effect
A) fluorescence
B) phosphorescence
C) photography
D) the photoelectric effect

Unlock Deck
Unlock for access to all 155 flashcards in this deck.
Unlock Deck
k this deck
14
Which of the following forms of radiation consists of positively charged particles?
A) α
B)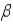
C)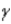
D) none of these
A) α
B)
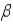
C)
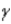
D) none of these

Unlock Deck
Unlock for access to all 155 flashcards in this deck.
Unlock Deck
k this deck
15
Radioactivity was first discovered in salts of which of the following elements?
A) sodium
B) radium
C) radon
D) uranium
A) sodium
B) radium
C) radon
D) uranium

Unlock Deck
Unlock for access to all 155 flashcards in this deck.
Unlock Deck
k this deck
16
Which of the following gives the correct order for the frequencies of portions of the electromagnetic spectrum?
A) radio > infrared > ultraviolet
B) radio > ultraviolet > infrared
C) ultraviolet > infrared > radio
D) ultraviolet > radio > infrared
A) radio > infrared > ultraviolet
B) radio > ultraviolet > infrared
C) ultraviolet > infrared > radio
D) ultraviolet > radio > infrared

Unlock Deck
Unlock for access to all 155 flashcards in this deck.
Unlock Deck
k this deck
17
Which of the following is the relationship between the wavelength, λ, and frequency, ν, of electromagnetic radiation?
A) ν =cλ
B) ν =λ/c
C) ν =c/λ
D) ν =1/cλ
A) ν =cλ
B) ν =λ/c
C) ν =c/λ
D) ν =1/cλ

Unlock Deck
Unlock for access to all 155 flashcards in this deck.
Unlock Deck
k this deck
18
Which of the following scientists discovered X-rays?
A) H. Becquerel
B) M. Curie
C) A. Einstein
D) W. Roentgen
A) H. Becquerel
B) M. Curie
C) A. Einstein
D) W. Roentgen

Unlock Deck
Unlock for access to all 155 flashcards in this deck.
Unlock Deck
k this deck
19
Which of the following scientists discovered radioactivity?
A) H. Becquerel
B) M. Curie
C) A. Einstein
D) E. Rutherford
A) H. Becquerel
B) M. Curie
C) A. Einstein
D) E. Rutherford

Unlock Deck
Unlock for access to all 155 flashcards in this deck.
Unlock Deck
k this deck
20
Which of the following forms of radiation is identical to the nucleus of a helium atom?
A) α
B)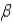
C)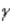
D) positron
A) α
B)
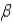
C)
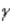
D) positron

Unlock Deck
Unlock for access to all 155 flashcards in this deck.
Unlock Deck
k this deck
21
What happens to the atomic number of an element when an α particle is emitted?
A) increases
B) decreases
C) remains constant
A) increases
B) decreases
C) remains constant

Unlock Deck
Unlock for access to all 155 flashcards in this deck.
Unlock Deck
k this deck
22
Which of the following statements is true?
A) In heavy atoms there are generally many more protons than neutrons.
B) In heavy atoms there are generally many more neutrons than protons.
C) In heavy atoms the number of protons and neutrons are nearly equal.
D) Most heavy atoms do not exist in a number of isotopic forms.
A) In heavy atoms there are generally many more protons than neutrons.
B) In heavy atoms there are generally many more neutrons than protons.
C) In heavy atoms the number of protons and neutrons are nearly equal.
D) Most heavy atoms do not exist in a number of isotopic forms.

Unlock Deck
Unlock for access to all 155 flashcards in this deck.
Unlock Deck
k this deck
23
Which of the following statements is true of electromagnetic radiation?
A) All electromagnetic radiation has the same energy.
B) As the wavelength of the radiation increases, the energy of the radiation decreases.
C) As the wavelength of the radiation increases, the energy of the radiation increases.
D) There is no specific relationship between wavelength and energy.
A) All electromagnetic radiation has the same energy.
B) As the wavelength of the radiation increases, the energy of the radiation decreases.
C) As the wavelength of the radiation increases, the energy of the radiation increases.
D) There is no specific relationship between wavelength and energy.

Unlock Deck
Unlock for access to all 155 flashcards in this deck.
Unlock Deck
k this deck
24
Which of the following is the name given to a packet of electromagnetic radiation?
A) phonon
B) phonton
C) phason
D) photon
A) phonon
B) phonton
C) phason
D) photon

Unlock Deck
Unlock for access to all 155 flashcards in this deck.
Unlock Deck
k this deck
25
Potassium-40 is a β emitter. What isotope is formed when 40K emits a β particle?
A) 36Cl
B) 40Ar
C) 40Ca
D) 44Ca
A) 36Cl
B) 40Ar
C) 40Ca
D) 44Ca

Unlock Deck
Unlock for access to all 155 flashcards in this deck.
Unlock Deck
k this deck
26
Which of the following gives the correct order for the wavelengths of portions of the electromagnetic spectrum?
A) radio > infrared > ultraviolet
B) radio > ultraviolet > infrared
C) ultraviolet > infrared > radio
D) ultraviolet > radio > infrared
A) radio > infrared > ultraviolet
B) radio > ultraviolet > infrared
C) ultraviolet > infrared > radio
D) ultraviolet > radio > infrared

Unlock Deck
Unlock for access to all 155 flashcards in this deck.
Unlock Deck
k this deck
27
Transmutation is caused by which of the following radioactive processes?
A) β emission
B) γ emission
C) both a and b
D) neither a nor b
A) β emission
B) γ emission
C) both a and b
D) neither a nor b

Unlock Deck
Unlock for access to all 155 flashcards in this deck.
Unlock Deck
k this deck
28
Carbon-14 is a β emitter. What isotope is formed when 14C emits a β particle?
A) 14B
B) 14N
C) 14O
D) 16O
A) 14B
B) 14N
C) 14O
D) 16O

Unlock Deck
Unlock for access to all 155 flashcards in this deck.
Unlock Deck
k this deck
29
Which of the following statements is true?
A) In light atoms there are generally many more protons than neutrons.
B) In light atoms there are generally many more neutrons than protons.
C) In light atoms the number of protons and neutrons are nearly equal.
D) Most naturally occurring isotopes of light atoms are radioactive.
A) In light atoms there are generally many more protons than neutrons.
B) In light atoms there are generally many more neutrons than protons.
C) In light atoms the number of protons and neutrons are nearly equal.
D) Most naturally occurring isotopes of light atoms are radioactive.

Unlock Deck
Unlock for access to all 155 flashcards in this deck.
Unlock Deck
k this deck
30
Which isotope of hydrogen is radioactive?
A) 1H
B) 2H
C) 3H
D) none of these
A) 1H
B) 2H
C) 3H
D) none of these

Unlock Deck
Unlock for access to all 155 flashcards in this deck.
Unlock Deck
k this deck
31
What happens to the mass number of an element produced when an α particle is emitted?
A) increases
B) decreases
C) remains constant
A) increases
B) decreases
C) remains constant

Unlock Deck
Unlock for access to all 155 flashcards in this deck.
Unlock Deck
k this deck
32
Which of the following radioactive processes does not result in transmutation?
A) α emission
B) β emission
C) γ emission
D) all of the above
A) α emission
B) β emission
C) γ emission
D) all of the above

Unlock Deck
Unlock for access to all 155 flashcards in this deck.
Unlock Deck
k this deck
33
Which of the following statements is true of electromagnetic radiation?
A) All electromagnetic radiation has the same energy.
B) As the frequency of the radiation increases, the energy of the radiation decreases.
C) As the frequency of the radiation increases, the energy of the radiation increases.
D) There is no specific relationship between frequency and energy.
A) All electromagnetic radiation has the same energy.
B) As the frequency of the radiation increases, the energy of the radiation decreases.
C) As the frequency of the radiation increases, the energy of the radiation increases.
D) There is no specific relationship between frequency and energy.

Unlock Deck
Unlock for access to all 155 flashcards in this deck.
Unlock Deck
k this deck
34
Approximately how many stable isotopes have been identified?
A) 92
B) 118
C) 264
D) more than 300
A) 92
B) 118
C) 264
D) more than 300

Unlock Deck
Unlock for access to all 155 flashcards in this deck.
Unlock Deck
k this deck
35
With which part of the atom is radioactivity associated?
A) the core electrons
B) the entire atom
C) the nucleus
D) the valence electrons
A) the core electrons
B) the entire atom
C) the nucleus
D) the valence electrons

Unlock Deck
Unlock for access to all 155 flashcards in this deck.
Unlock Deck
k this deck
36
How many artificial radioisotopes have been synthesized in the laboratory?
A) 26
B) 92
C) approximately 500
D) more than 1000
A) 26
B) 92
C) approximately 500
D) more than 1000

Unlock Deck
Unlock for access to all 155 flashcards in this deck.
Unlock Deck
k this deck
37
Transmutation is caused by which of the following radioactive processes?
A) α emission
B) γ emission
C) both a and b
D) neither a nor b
A) α emission
B) γ emission
C) both a and b
D) neither a nor b

Unlock Deck
Unlock for access to all 155 flashcards in this deck.
Unlock Deck
k this deck
38
Which of the following statements is true of artificially created isotopes?
A) All artificially created isotopes are stable.
B) All artificially created isotopes are unstable.
C) Most artificially created isotopes are stable.
D) Most artificially created isotopes are unstable.
A) All artificially created isotopes are stable.
B) All artificially created isotopes are unstable.
C) Most artificially created isotopes are stable.
D) Most artificially created isotopes are unstable.

Unlock Deck
Unlock for access to all 155 flashcards in this deck.
Unlock Deck
k this deck
39
Transmutation is caused by which of the following radioactive processes?
A) α emission
B) β emission
C) both a and b
D) neither a nor b
A) α emission
B) β emission
C) both a and b
D) neither a nor b

Unlock Deck
Unlock for access to all 155 flashcards in this deck.
Unlock Deck
k this deck
40
How many naturally occurring isotopes have been identified?
A) 92
B) 118
C) fewer than 300
D) more than 300
A) 92
B) 118
C) fewer than 300
D) more than 300

Unlock Deck
Unlock for access to all 155 flashcards in this deck.
Unlock Deck
k this deck
41
Which isotope of oxygen is most likely to be a positron emitter?
A) 15O
B) 16O
C) 17O
D) 19O
A) 15O
B) 16O
C) 17O
D) 19O

Unlock Deck
Unlock for access to all 155 flashcards in this deck.
Unlock Deck
k this deck
42
Thorium-223 is an α emitter. What isotope is formed when 223Th emits an α particle?
A) 221Fr
B) 223Pa
C) 219Po
D) 219Ra
A) 221Fr
B) 223Pa
C) 219Po
D) 219Ra

Unlock Deck
Unlock for access to all 155 flashcards in this deck.
Unlock Deck
k this deck
43
Thallium-201 decays by electron capture. What isotope is formed when 201Tl decays in this way?
A) 201Au
B) 201Hg
C) 201Pb
D) 200Tl
A) 201Au
B) 201Hg
C) 201Pb
D) 200Tl

Unlock Deck
Unlock for access to all 155 flashcards in this deck.
Unlock Deck
k this deck
44
Which isotope of phosphorous is most likely to be a positron emitter?
A) 29P
B) 30P
C) 31P
D) 32P
A) 29P
B) 30P
C) 31P
D) 32P

Unlock Deck
Unlock for access to all 155 flashcards in this deck.
Unlock Deck
k this deck
45
Chromium-51 decays by electron capture. What isotope is formed when 51Cr decays in this way?
A) 50Cr
B) 52Cr
C) 51Mn
D) 51V
A) 50Cr
B) 52Cr
C) 51Mn
D) 51V

Unlock Deck
Unlock for access to all 155 flashcards in this deck.
Unlock Deck
k this deck
46
What is the mass number of the element produced when an element with mass number A emits a positron?
A) A __ 2
B) A __ 1
C) A
D) A + 1
A) A __ 2
B) A __ 1
C) A
D) A + 1

Unlock Deck
Unlock for access to all 155 flashcards in this deck.
Unlock Deck
k this deck
47
Cobalt-60 is a γ emitter. What isotope is formed when 60Co emits γ radiation?
A) 60Mn
B) 60Fe
C) 60Co
D) 60Ni
A) 60Mn
B) 60Fe
C) 60Co
D) 60Ni

Unlock Deck
Unlock for access to all 155 flashcards in this deck.
Unlock Deck
k this deck
48
Which type of radioactive particles has the longest half-life?
A) α emitters
B) β emitters
C) γ emitters
D) There is no relationship between half-life and the type of radiation.
A) α emitters
B) β emitters
C) γ emitters
D) There is no relationship between half-life and the type of radiation.

Unlock Deck
Unlock for access to all 155 flashcards in this deck.
Unlock Deck
k this deck
49
Which of the following affects the half-life of a radioactive sample?
A) the age of sample
B) the size of the sample
C) the temperature of the sample
D) none of these
A) the age of sample
B) the size of the sample
C) the temperature of the sample
D) none of these

Unlock Deck
Unlock for access to all 155 flashcards in this deck.
Unlock Deck
k this deck
50
What is the mass number of the element produced when an element with mass number A emits an α particle?
A) A __ 4
B) A __ 2
C) A
D) A + 2
A) A __ 4
B) A __ 2
C) A
D) A + 2

Unlock Deck
Unlock for access to all 155 flashcards in this deck.
Unlock Deck
k this deck
51
Polonium-210 is an α emitter. What isotope is formed when 210Po emits an α particle?
A) 210At
B) 206Pb
C) 206Rn
D) 208Xe
A) 210At
B) 206Pb
C) 206Rn
D) 208Xe

Unlock Deck
Unlock for access to all 155 flashcards in this deck.
Unlock Deck
k this deck
52
What is the atomic number of the element produced when an element with atomic number Z emits an α particle?
A) Z __ 2
B) Z __ 1
C) Z + 1
D) Z + 2
A) Z __ 2
B) Z __ 1
C) Z + 1
D) Z + 2

Unlock Deck
Unlock for access to all 155 flashcards in this deck.
Unlock Deck
k this deck
53
What is the atomic number of the element produced when an element with atomic number Z undergoes electron capture?
A) Z __ 2
B) Z __ 1
C) Z + 1
D) Z + 2
A) Z __ 2
B) Z __ 1
C) Z + 1
D) Z + 2

Unlock Deck
Unlock for access to all 155 flashcards in this deck.
Unlock Deck
k this deck
54
Which isotope of oxygen is most likely to be a β emitter?
A) 15O
B) 16O
C) 17O
D) 19O
A) 15O
B) 16O
C) 17O
D) 19O

Unlock Deck
Unlock for access to all 155 flashcards in this deck.
Unlock Deck
k this deck
55
Beryllium-7 decays by electron capture. What isotope is formed when 7Be decays in this way?
A) 7B
B) 6Be
C) 8Be
D) 7Li
A) 7B
B) 6Be
C) 8Be
D) 7Li

Unlock Deck
Unlock for access to all 155 flashcards in this deck.
Unlock Deck
k this deck
56
Which isotope of phosphorous is most likely to be a β emitter?
A) 29P
B) 30P
C) 31P
D) 32P
A) 29P
B) 30P
C) 31P
D) 32P

Unlock Deck
Unlock for access to all 155 flashcards in this deck.
Unlock Deck
k this deck
57
Tritium, 3H, is a β emitter. What isotope is formed when 3H emits a β particle?
A) 1H
B) 2H
C) 3He
D) 4He
A) 1H
B) 2H
C) 3He
D) 4He

Unlock Deck
Unlock for access to all 155 flashcards in this deck.
Unlock Deck
k this deck
58
What is the atomic number of the element produced when an element with atomic number Z emits a positron?
A) Z __ 2
B) Z __ 1
C) Z + 1
D) Z + 2
A) Z __ 2
B) Z __ 1
C) Z + 1
D) Z + 2

Unlock Deck
Unlock for access to all 155 flashcards in this deck.
Unlock Deck
k this deck
59
Arsenic-74 is a positron emitter. What isotope is formed when 74As emits a positron?
A) 75Br
B) 70Ga
C) 74Ge
D) 74Se
A) 75Br
B) 70Ga
C) 74Ge
D) 74Se

Unlock Deck
Unlock for access to all 155 flashcards in this deck.
Unlock Deck
k this deck
60
Mercury-197 is a γ emitter. What isotope is formed when 197Hg emits γ radiation?
A) 197Au
B) 197Hg
C) 197Tl
D) 197Pb
A) 197Au
B) 197Hg
C) 197Tl
D) 197Pb

Unlock Deck
Unlock for access to all 155 flashcards in this deck.
Unlock Deck
k this deck
61
Radiation detectors use which of the following properties to detect radioactivity?
A) charge
B) ionizing ability
C) mass
D) all of these
A) charge
B) ionizing ability
C) mass
D) all of these

Unlock Deck
Unlock for access to all 155 flashcards in this deck.
Unlock Deck
k this deck
62
The half-life of I-131 is 8.0 days. If a medical treatment involves a dose of 100. mg of I-131, how much of the isotope remains after 16 days?
A) 12.5 mg
B) 25.0 mg
C) 50.0 mg
D) 75.0 mg
A) 12.5 mg
B) 25.0 mg
C) 50.0 mg
D) 75.0 mg

Unlock Deck
Unlock for access to all 155 flashcards in this deck.
Unlock Deck
k this deck
63
What kinds of ions are produced by ionizing radiation?
A) only positively charged ions
B) only negatively charged ions
C) both positively charged and negatively ions
D) The type of ions produced depend on the type of ionizing radiation.
A) only positively charged ions
B) only negatively charged ions
C) both positively charged and negatively ions
D) The type of ions produced depend on the type of ionizing radiation.

Unlock Deck
Unlock for access to all 155 flashcards in this deck.
Unlock Deck
k this deck
64
Which of the following correctly relates the becquerel to the curie?
A) 3.7 × 1010 Bq = 1 Ci
B) 3.7 × 107 Bq = 1 Ci
C) 2.7 × 10-8 Bq = 1 Ci
D) 2.7 × 10-11 Bq = 1 Ci
A) 3.7 × 1010 Bq = 1 Ci
B) 3.7 × 107 Bq = 1 Ci
C) 2.7 × 10-8 Bq = 1 Ci
D) 2.7 × 10-11 Bq = 1 Ci

Unlock Deck
Unlock for access to all 155 flashcards in this deck.
Unlock Deck
k this deck
65
Which of our senses can we use to detect nuclear radiation?
A) hearing
B) sight
C) taste
D) none
A) hearing
B) sight
C) taste
D) none

Unlock Deck
Unlock for access to all 155 flashcards in this deck.
Unlock Deck
k this deck
66
What percentage of a radioactive sample remains after 4 half-lives?
A) 25.0%
B) 12.5%
C) 6.25%
D) 3.13%
A) 25.0%
B) 12.5%
C) 6.25%
D) 3.13%

Unlock Deck
Unlock for access to all 155 flashcards in this deck.
Unlock Deck
k this deck
67
Krypton-85 has a half-life of 10 years. Approximately what percentage of Kr-85 produced from nuclear testing in 1956 still remains radioactive today?
A) 25%
B) 12%
C) 6%
D) 3%
A) 25%
B) 12%
C) 6%
D) 3%

Unlock Deck
Unlock for access to all 155 flashcards in this deck.
Unlock Deck
k this deck
68
Different isotopes are useful in different dating processes. Suppose we wished to determine the age of an object which we suspect to be approximately 10,000 years old. An isotope with which of the following half-lives would be most appropriate for this project?
A) 50 years
B) 500 years
C) 5,000 years
D) 50,000 years
A) 50 years
B) 500 years
C) 5,000 years
D) 50,000 years

Unlock Deck
Unlock for access to all 155 flashcards in this deck.
Unlock Deck
k this deck
69
mg
C) 50.0 mg
D) 25.0 mg
C) 50.0 mg
D) 25.0 mg

Unlock Deck
Unlock for access to all 155 flashcards in this deck.
Unlock Deck
k this deck
70
In which of the following radiation detectors is a phosphor used?
A) a Geiger-Müller counter
B) a proportional counter
C) a scintillation counter
D) all of these
A) a Geiger-Müller counter
B) a proportional counter
C) a scintillation counter
D) all of these

Unlock Deck
Unlock for access to all 155 flashcards in this deck.
Unlock Deck
k this deck
71
Isotopes with which of the following half-lives are most useful in medical therapy?
A) seconds to minutes
B) minutes to hours
C) hours to days
D) days to years
A) seconds to minutes
B) minutes to hours
C) hours to days
D) days to years

Unlock Deck
Unlock for access to all 155 flashcards in this deck.
Unlock Deck
k this deck
72
Barium-122 has a half-life of 2 minutes. If 10.0 g of Ba-122 are produced in a nuclear reactor how much Ba-122 will remain 10 minutes after production ceases?
A) 2.50 g
B) 1.25 g
C) 0.625 g
D) 0.313 g
A) 2.50 g
B) 1.25 g
C) 0.625 g
D) 0.313 g

Unlock Deck
Unlock for access to all 155 flashcards in this deck.
Unlock Deck
k this deck
73
Which of the following is the SI unit of radiation activity?
A) the becquerel
B) the curie
C) the millicurie
D) the roentgen
A) the becquerel
B) the curie
C) the millicurie
D) the roentgen

Unlock Deck
Unlock for access to all 155 flashcards in this deck.
Unlock Deck
k this deck
74
Ionizing radiation is characterized by which of the following?
A) its energy
B) its intensity
C) both a and b
D) neither a nor b
A) its energy
B) its intensity
C) both a and b
D) neither a nor b

Unlock Deck
Unlock for access to all 155 flashcards in this deck.
Unlock Deck
k this deck
75
Which of the following isotopes can be used by geologists to date rocks?
A) C-14
B) I-131
C) Sr-90
D) U-238
A) C-14
B) I-131
C) Sr-90
D) U-238

Unlock Deck
Unlock for access to all 155 flashcards in this deck.
Unlock Deck
k this deck
76
Krypton-85 has a half-life of 10 years. Approximately what percentage of Kr-85 produced from the Chernobyl accident in 1986 still remains radioactive in 2010?
A) 25%
B) 12%
C) 6%
D) 3%
A) 25%
B) 12%
C) 6%
D) 3%

Unlock Deck
Unlock for access to all 155 flashcards in this deck.
Unlock Deck
k this deck
77
Strontium-90 is a dangerous isotope because strontium is chemically similar to calcium and is easily incorporated into bone. Sr-90 has a half-life 28.1 years. If a person ingests 100. mg of Sr-90 how much will be left in the person's body after 56.2 years?
A) 75.0 mg
B) 50.0 mg
C) 25.0 mg
D) 12.5 mg
A) 75.0 mg
B) 50.0 mg
C) 25.0 mg
D) 12.5 mg

Unlock Deck
Unlock for access to all 155 flashcards in this deck.
Unlock Deck
k this deck
78
In which of the following radiation detectors is a gas ionized?
A) a Geiger-Müller counter
B) a scintillation counter
C) both a and b
D) neither a nor b
A) a Geiger-Müller counter
B) a scintillation counter
C) both a and b
D) neither a nor b

Unlock Deck
Unlock for access to all 155 flashcards in this deck.
Unlock Deck
k this deck
79
Different isotopes are useful in different dating processes. Suppose we wished to determine the age of an object which we suspect to be approximately 100 years old. An isotope with which of the following half-lives would be most appropriate for this project?
A) 5 years
B) 50 years
C) 500 years
D) 5,000 years
A) 5 years
B) 50 years
C) 500 years
D) 5,000 years

Unlock Deck
Unlock for access to all 155 flashcards in this deck.
Unlock Deck
k this deck
80
What percentage of a radioactive sample remains after 5 half-lives?
A) 6.25%
B) 3.13%
C) 1.56%
D) 0.781%
A) 6.25%
B) 3.13%
C) 1.56%
D) 0.781%

Unlock Deck
Unlock for access to all 155 flashcards in this deck.
Unlock Deck
k this deck



