Deck 10: Organic Chemistry
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/70
Play
Full screen (f)
Deck 10: Organic Chemistry
1
Approximately how many new organic compounds are reported each year?
A) 1000
B) 10,000
C) 100,000
D) 1,000,000
A) 1000
B) 10,000
C) 100,000
D) 1,000,000
B
2
Which element accounts for the largest percentage of the earth's crust?
A) carbon
B) nitrogen
C) oxygen
D) silicon
A) carbon
B) nitrogen
C) oxygen
D) silicon
C
3
If the bond angle at a carbon atom is 180°, how many groups are distributed around the carbon atom?
A) 1
B) 2
C) 3
D) 4
A) 1
B) 2
C) 3
D) 4
B
4
Which of the following statements about organic compounds is true?
A) Only naturally occurring compounds are considered organic compounds.
B) The number of naturally occurring compounds and the number of synthetic compounds are approximately equal.
C) The number of naturally occurring compounds greatly exceeds the number of synthetic compounds.
D) The number of naturally occurring compounds is much smaller than the number of synthetic compounds.
A) Only naturally occurring compounds are considered organic compounds.
B) The number of naturally occurring compounds and the number of synthetic compounds are approximately equal.
C) The number of naturally occurring compounds greatly exceeds the number of synthetic compounds.
D) The number of naturally occurring compounds is much smaller than the number of synthetic compounds.

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
5
The chemical formula of methylamine is CH3NH2. How many valence electrons are associated with a molecule of methylamine?
A) 7
B) 8
C) 12
D) 14
A) 7
B) 8
C) 12
D) 14

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
6
Chemicals in which of the following groups are not considered organic chemicals?
A) enzymes
B) minerals
C) proteins
D) vitamins
A) enzymes
B) minerals
C) proteins
D) vitamins

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
7
Which of the following is generally true of organic compounds?
A) they are typically high melting solids
B) most are soluble in water
C) both a and b
D) neither a nor b
A) they are typically high melting solids
B) most are soluble in water
C) both a and b
D) neither a nor b

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
8
Which of the following statements about taxol is false?
A) About 1 gram can be obtained from a 100 year old tree.
B) It is effective in treating certain ovarian and breast cancers.
C) Taxol synthesized in the laboratory is identical to naturally obtained taxol.
D) None, all of the above are true.
A) About 1 gram can be obtained from a 100 year old tree.
B) It is effective in treating certain ovarian and breast cancers.
C) Taxol synthesized in the laboratory is identical to naturally obtained taxol.
D) None, all of the above are true.

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
9
If the bond angles at a carbon atom are 120°, how many groups are distributed around the carbon atom?
A) 1
B) 2
C) 3
D) 4
A) 1
B) 2
C) 3
D) 4

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
10
Which of the following is generally true of most organic compounds?
A) their bonding is covalent in nature
B) their aqueous solutions conduct electricity
C) both a and b
D) neither a nor b
A) their bonding is covalent in nature
B) their aqueous solutions conduct electricity
C) both a and b
D) neither a nor b

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
11
Which of the following statements is false?
A) Organic chemistry involves the study of compounds which contain carbon.
B) Organic compounds cannot be synthesized in the laboratory.
C) The most common elements found in organic compounds are carbon, hydrogen, nitrogen, and oxygen.
D) Organic and inorganic compounds obey the same natural laws.
A) Organic chemistry involves the study of compounds which contain carbon.
B) Organic compounds cannot be synthesized in the laboratory.
C) The most common elements found in organic compounds are carbon, hydrogen, nitrogen, and oxygen.
D) Organic and inorganic compounds obey the same natural laws.

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
12
In organic compounds, which of the following elements normally forms two covalent bonds?
A) carbon
B) hydrogen
C) nitrogen
D) oxygen
A) carbon
B) hydrogen
C) nitrogen
D) oxygen

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
13
A chemist has two pure samples of an organic compound, one of which she obtained from a natural source, and one of which she synthesized in the lab. Which of the following properties can she use to determine which is the synthetic sample?
A) boiling point
B) formula weight
C) solubility
D) none of these
A) boiling point
B) formula weight
C) solubility
D) none of these

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
14
Which of the following is true of organic compounds?
A) most are gases, liquids, or low melting solids
B) they represent only 5% of all known compounds
C) both a and b
D) neither a nor b
A) most are gases, liquids, or low melting solids
B) they represent only 5% of all known compounds
C) both a and b
D) neither a nor b

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
15
The bond angles around the carbon atom in methane (CH4) are all:
A) 180o
B) 120o
C) 109.5o
D) 90o
A) 180o
B) 120o
C) 109.5o
D) 90o

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
16
Which of the following chemicals, when pure, will have different properties if it is synthesized in the laboratory than if it is obtained from a natural source?
A) ethanol
B) taxol
C) vitamin C
D) none of these
A) ethanol
B) taxol
C) vitamin C
D) none of these

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
17
Which of the following statements about taxol is false?
A) Taxol is no longer obtained primarily from the bark of the Pacific yew.
B) Taxol and paclitaxel are two different but related anticancer drugs.
C) Taxol has been successfully synthesized by chemists.
D) Taxol inhibits cell division by preventing the disassembly of microtubules.
A) Taxol is no longer obtained primarily from the bark of the Pacific yew.
B) Taxol and paclitaxel are two different but related anticancer drugs.
C) Taxol has been successfully synthesized by chemists.
D) Taxol inhibits cell division by preventing the disassembly of microtubules.

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
18
In organic compounds, which of the following elements normally forms three covalent bonds?
A) carbon
B) hydrogen
C) nitrogen
D) oxygen
A) carbon
B) hydrogen
C) nitrogen
D) oxygen

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
19
If the bond angles at a carbon atom are 109.5°, how many groups are distributed around the carbon atom?
A) 1
B) 2
C) 3
D) 4
A) 1
B) 2
C) 3
D) 4

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
20
Which of the following geometries is never associated with a carbon atom in an organic compound?
A) linear
B) square planar
C) tetrahedral
D) trigonal planar
A) linear
B) square planar
C) tetrahedral
D) trigonal planar

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
21
The 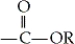 group is the functional group of which of the following types of compounds?
group is the functional group of which of the following types of compounds?
A) carboxylic acids
B) aldehydes
C) carboxylic esters
D) ketones
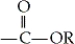 group is the functional group of which of the following types of compounds?
group is the functional group of which of the following types of compounds?A) carboxylic acids
B) aldehydes
C) carboxylic esters
D) ketones

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
22
In which of the following classes of organic compounds is an oxygen atom bonded to a hydrogen atom?
A) aldehydes
B) carboxylic acids
C) ketones
D) all of these
A) aldehydes
B) carboxylic acids
C) ketones
D) all of these

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
23
In which of the following classes of organic compounds is an oxygen atom bonded to a hydrogen atom?
A) alcohols
B) aldehydes
C) amines
D) all of these
A) alcohols
B) aldehydes
C) amines
D) all of these

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
24
What is the minimum number of hydrogen atoms present in a carboxylic acid?
A) 1
B) 2
C) 3
D) 4
A) 1
B) 2
C) 3
D) 4

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
25
Which of the following classes of compounds can be categorized as primary, secondary, or tertiary?
A) alcohols
B) aldehydes
C) carboxylic acids
D) ketones
A) alcohols
B) aldehydes
C) carboxylic acids
D) ketones

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
26
Which of the following functional groups does not contain a carbonyl group?
A) ester
B) alcohol
C) aldehyde
D) ketone
A) ester
B) alcohol
C) aldehyde
D) ketone

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
27
The chemical formula of methanol is CH3OH. How many unshared electron pairs are associated with a molecule of methanol?
A) 1
B) 2
C) 4
D) 6
A) 1
B) 2
C) 4
D) 6

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
28
Amino acids are molecules which contain both an amino group and a carboxyl group. Which atomic species must be present in all amino acids?
A) C and H
B) C, H, and N
C) C, H, and O
D) C, H, N, and O
A) C and H
B) C, H, and N
C) C, H, and O
D) C, H, N, and O

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
29
In which of the following classes of organic compounds must every compound contain more than one carbon atom?
A) alcohols
B) aldehydes
C) carboxylic acids
D) ketones
A) alcohols
B) aldehydes
C) carboxylic acids
D) ketones

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
30
Of the four alcohols with molecular formula C4H10O, how many are primary alcohols?
A) 1
B) 2
C) 3
D) 4
A) 1
B) 2
C) 3
D) 4

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
31
Which of the following statements is false?
A) In a secondary amine, the nitrogen is bonded to two carbon atoms.
B) In a tertiary alcohol, the carbon bearing the OH group is bonded to no hydrogen atoms.
C) Aldehydes, ketones, and esters all contain a carbonyl group.
D) The molecular formula for the smallest carboxylic acid is C2H4O2.
A) In a secondary amine, the nitrogen is bonded to two carbon atoms.
B) In a tertiary alcohol, the carbon bearing the OH group is bonded to no hydrogen atoms.
C) Aldehydes, ketones, and esters all contain a carbonyl group.
D) The molecular formula for the smallest carboxylic acid is C2H4O2.

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
32
In which of the following classes of organic compounds must there be at least two oxygen atoms?
A) carboxylic esters
B) ketones
C) both a and b
D) neither a nor b
A) carboxylic esters
B) ketones
C) both a and b
D) neither a nor b

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
33
The chemical formula of hydrogen cyanide is HCN. How many unshared electrons are associated with a molecule of hydrogen cyanide?
A) 2
B) 4
C) 6
D) 10
A) 2
B) 4
C) 6
D) 10

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
34
In which of the following classes of organic compounds must there be at least two oxygen atoms?
A) alcohols
B) aldehydes
C) carboxylic acids
D) ketones
A) alcohols
B) aldehydes
C) carboxylic acids
D) ketones

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
35
In which of the following classes of organic compounds must there be at least two oxygen atoms?
A) carboxylic acids
B) carboxylic esters
C) both a and b
D) neither a nor b
A) carboxylic acids
B) carboxylic esters
C) both a and b
D) neither a nor b

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
36
The chemical formula of hydrogen cyanide is HCN. How many valence electrons are associated with a molecule of hydrogen cyanide?
A) 4
B) 6
C) 10
D) 14
A) 4
B) 6
C) 10
D) 14

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
37
Which of the following classes of compounds can be categorized as primary, secondary or, tertiary?
A) aldehydes
B) amines
C) carboxylic acids
D) ketones
A) aldehydes
B) amines
C) carboxylic acids
D) ketones

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
38
The chemical formula of acetylene is C2H2. How many valence electrons are associated with a molecule of acetylene?
A) 4
B) 5
C) 8
D) 10
A) 4
B) 5
C) 8
D) 10

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
39
What is the minimum number of hydrogen atoms present in a carboxylic ester?
A) 1
B) 2
C) 4
D) 6
A) 1
B) 2
C) 4
D) 6

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
40
The chemical formula of methylamine is CH3NH2. How many unshared electron pairs are associated with a molecule of methylamine?
A) 1
B) 2
C) 8
D) 14
A) 1
B) 2
C) 8
D) 14

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
41
Which of the following is an example of a secondary alcohol?
A) CH3OH
B) CH3CH2OH
C) CH3CH2CH2OH
D) none of these
A) CH3OH
B) CH3CH2OH
C) CH3CH2CH2OH
D) none of these

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
42
How many amines have the molecular formula C3H9N?
A) 1
B) 2
C) 3
D) 4
A) 1
B) 2
C) 3
D) 4

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
43
Which of the following is an example of a carboxylic acid?
A) CH3CH2OH
B)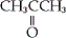
C)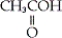
D)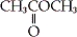
A) CH3CH2OH
B)
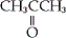
C)
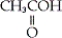
D)
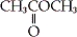

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
44
How many aldehydes have the molecular formula C4H8O?
A) 1
B) 2
C) 3
D) 4
A) 1
B) 2
C) 3
D) 4

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
45
Which of the following is an example of a tertiary amine?
A) CH3NH2
B) CH3CH2NH2
C) CH3CH2CH2NH2
D) none of these
A) CH3NH2
B) CH3CH2NH2
C) CH3CH2CH2NH2
D) none of these

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
46
How many ketones have the molecular formula C5H10O?
A) 1
B) 2
C) 3
D) 4
A) 1
B) 2
C) 3
D) 4

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
47
Which of the following is an example of a secondary alcohol?
A) CH3OH
B)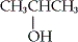
C) CH3CH2OH
D) none of these
A) CH3OH
B)
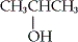
C) CH3CH2OH
D) none of these

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
48
How many carboxylic esters have the molecular formula C3H6O2?
A) 1
B) 2
C) 3
D) 4
A) 1
B) 2
C) 3
D) 4

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
49
Which of the following is an example of a secondary amine?
A) CH3NH2
B)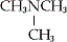
C)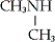
D) none of these
A) CH3NH2
B)
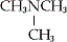
C)
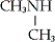
D) none of these

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
50
Which of the following is an example of a tertiary alcohol?
A) CH3OH
B) CH3CH2OH
C) CH3CH2CH2OH
D) none of these
A) CH3OH
B) CH3CH2OH
C) CH3CH2CH2OH
D) none of these

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
51
How many secondary amines have the molecular formula C4H11N?
A) 1
B) 2
C) 3
D) 4
A) 1
B) 2
C) 3
D) 4

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
52
How many carboxylic acids have the molecular formula C4H8O2?
A) 1
B) 2
C) 3
D) 4
A) 1
B) 2
C) 3
D) 4

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
53
Which of the following is an example of a tertiary amine?
A) CH3NH2
B)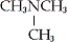
C)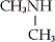
D) none of these
A) CH3NH2
B)
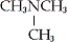
C)
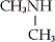
D) none of these

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
54
Which of the following is an example of a carboxylic acid?
A)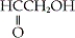
B)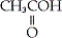
C) both a and b
D) neither a nor b
A)
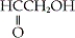
B)
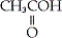
C) both a and b
D) neither a nor b

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
55
Which of the following is an example of a secondary amine?
A) CH3NH2
B) CH3CH2NH2
C) CH3CH2NHCH3
D) none of these
A) CH3NH2
B) CH3CH2NH2
C) CH3CH2NHCH3
D) none of these

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
56
Which of the following is an example of a carboxylic acid?
A)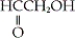
B)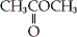
C) both a and b
D) neither a nor b
A)
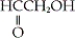
B)
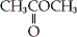
C) both a and b
D) neither a nor b

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
57
Which of the following is an example of a carboxylic ester?
A) CH3COOH
B) CH3CH2OH
C) CH3CHO
D)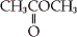
A) CH3COOH
B) CH3CH2OH
C) CH3CHO
D)
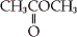

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
58
Which of the following is an example of a secondary amine?
A) CH3CH2NH2
B) CH3CH2CH2NHCH3
C)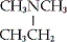
D) none of these
A) CH3CH2NH2
B) CH3CH2CH2NHCH3
C)
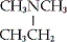
D) none of these

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
59
Which of the following is an example of a primary amine?
A) CH3NH2
B) CH3CH2NH2
C) CH3CH2CH2NH2
D) all of these
A) CH3NH2
B) CH3CH2NH2
C) CH3CH2CH2NH2
D) all of these

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
60
Which of the following is an example of a secondary amine?
A) CH3NH2
B) CH3CH2NH2
C) CH3CH2CH2NH2
D) none of these
A) CH3NH2
B) CH3CH2NH2
C) CH3CH2CH2NH2
D) none of these

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
61
Examine the following models. Atoms other than carbon and hydrogen are labeled with the symbol of the element. 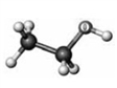
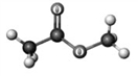
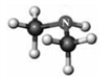 A B C D
A B C D
Which model represents a derivative of a carboxylic acid?
A) A
B) B
C) C
D) D
E) None of these is a derivative of a carboxylic acid.



 A B C D
A B C DWhich model represents a derivative of a carboxylic acid?
A) A
B) B
C) C
D) D
E) None of these is a derivative of a carboxylic acid.

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
62
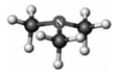
Examine the following models. Atoms other than carbon and hydrogen are labeled with the symbol of the element. 


 A B C D
A B C D
Which model represents a secondary amine?
A) A
B) B
C) C
D) D
E) None of these is a secondary amine.



 A B C D
A B C DWhich model represents a secondary amine?
A) A
B) B
C) C
D) D
E) None of these is a secondary amine.

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
63
Examine the following models. Atoms other than carbon and hydrogen are labeled with the symbol of the element. 


 A B C D
A B C D
Which model represents an ester?
A) A
B) B
C) C
D) D
E) None of these is an ester..



 A B C D
A B C DWhich model represents an ester?
A) A
B) B
C) C
D) D
E) None of these is an ester..

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
64
Examine the following models. Atoms other than carbon and hydrogen are labeled with the symbol of the element. 


 A B C D
A B C D
Which of the models is in the same functional group as the compound represented below? Atoms other than carbon and hydrogen are labeled with the symbol of the element.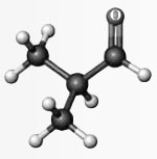
A) A
B) B
C) C
D) D
E) None of these is in the same functional group.



 A B C D
A B C DWhich of the models is in the same functional group as the compound represented below? Atoms other than carbon and hydrogen are labeled with the symbol of the element.

A) A
B) B
C) C
D) D
E) None of these is in the same functional group.

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
65
Examine the following structure. Atoms other than carbon and hydrogen are labeled with the symbol of the element. 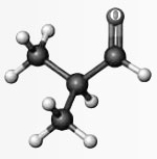
What are the bond angles around the carbon atom bonded to the oxygen atom?
A) 109.5°
B) 120°
C) 180°
D) 90°

What are the bond angles around the carbon atom bonded to the oxygen atom?
A) 109.5°
B) 120°
C) 180°
D) 90°

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
66
Examine the following models. Atoms other than carbon and hydrogen are labeled with the symbol of the element. 


 A B C D
A B C D
Which of the models could be represented by the following condensed formula? CH3COOCH3
A) A
B) B
C) C
D) D
E) None of these is a derivative of a carboxylic acid.



 A B C D
A B C DWhich of the models could be represented by the following condensed formula? CH3COOCH3
A) A
B) B
C) C
D) D
E) None of these is a derivative of a carboxylic acid.

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
67
Which of the following is an example a carboxylic ester?
A) CH3CH2OH
B)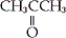
C)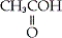
D)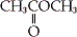
A) CH3CH2OH
B)
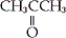
C)
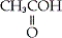
D)
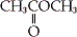

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
68
Examine the following structure. Atoms other than carbon and hydrogen are labeled with the symbol of the element. 
What type of functional group is present in this compound?
A) ketone
B) aldehyde
C) ester
D) carboxylic acid

What type of functional group is present in this compound?
A) ketone
B) aldehyde
C) ester
D) carboxylic acid

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
69
Examine the following models. Atoms other than carbon and hydrogen are labeled with the symbol of the element. 


 A B C D
A B C D
Which model represents a secondary alcohol?
A) A
B) B
C) C
D) D
E) None of these is a secondary alcohol.



 A B C D
A B C DWhich model represents a secondary alcohol?
A) A
B) B
C) C
D) D
E) None of these is a secondary alcohol.

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
70
Which of the following is an example a carboxylic ester?
A)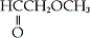
B)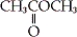
C) both a and b
D) neither a nor b
A)
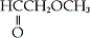
B)
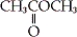
C) both a and b
D) neither a nor b

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck



