Deck 8: Acids and Bases
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/197
Play
Full screen (f)
Deck 8: Acids and Bases
1
When solid sodium hydroxide is dissolved in water which of the following species is not present in the solution?
A) H2O(l)
B) Na+(aq)
C) OH-(aq)
D) None, they are all present.
A) H2O(l)
B) Na+(aq)
C) OH-(aq)
D) None, they are all present.
D
2
The species H3O+ can be called which of the following?
A) heavy water
B) hydrogen ion
C) hydronium ion
D) all of these
A) heavy water
B) hydrogen ion
C) hydronium ion
D) all of these
C
3
When solid sodium hydroxide is dissolved in water which of the following species is not present in the solution?
A) NaOH(aq)
B) H2O(l)
C) OH-(aq)
D) None, they are all present.
A) NaOH(aq)
B) H2O(l)
C) OH-(aq)
D) None, they are all present.
A
4
Which of the following can be characterized as a strong base?
A) LiOH
B) KOH
C) both a and b
D) neither a nor b
A) LiOH
B) KOH
C) both a and b
D) neither a nor b

Unlock Deck
Unlock for access to all 197 flashcards in this deck.
Unlock Deck
k this deck
5
Which of the following can be characterized as a strong acid?
A) 0.001 M HCl
B) 3.0 M HCl
C) both a and b
D) neither a nor b
A) 0.001 M HCl
B) 3.0 M HCl
C) both a and b
D) neither a nor b

Unlock Deck
Unlock for access to all 197 flashcards in this deck.
Unlock Deck
k this deck
6
When hydrogen chloride gas is dissolved in water which of the following species is not present in the solution?
A) HCl(aq)
B) H2O(l)
C) Cl-(aq)
D) None, they are all present.
A) HCl(aq)
B) H2O(l)
C) Cl-(aq)
D) None, they are all present.

Unlock Deck
Unlock for access to all 197 flashcards in this deck.
Unlock Deck
k this deck
7
According to Arrhenius which species is associated with all acids?
A) H3O+
B) OH-
C) H2O
D) none of these
A) H3O+
B) OH-
C) H2O
D) none of these

Unlock Deck
Unlock for access to all 197 flashcards in this deck.
Unlock Deck
k this deck
8
Which of the following can be characterized as a strong base?
A) Ba(OH)2
B) NH3
C) both a and b
D) neither a nor b
A) Ba(OH)2
B) NH3
C) both a and b
D) neither a nor b

Unlock Deck
Unlock for access to all 197 flashcards in this deck.
Unlock Deck
k this deck
9
Which of the following can be characterized as a strong acid?
A) HF
B) HCl
C) both a and b
D) neither a nor b
A) HF
B) HCl
C) both a and b
D) neither a nor b

Unlock Deck
Unlock for access to all 197 flashcards in this deck.
Unlock Deck
k this deck
10
Why does the label ammonium hydroxide on a bottle give a false impression about the bottle=s contents?
A) There is a large amount of NH4+(aq) but only a small amount of OH-(aq).
B) There is a small amount of NH4+(aq) but only a large amount of OH-(aq).
C) There is a large amount of NH3(aq) but only small amount of OH-(aq).
D) None of the above, the label accurately describes the contents.
A) There is a large amount of NH4+(aq) but only a small amount of OH-(aq).
B) There is a small amount of NH4+(aq) but only a large amount of OH-(aq).
C) There is a large amount of NH3(aq) but only small amount of OH-(aq).
D) None of the above, the label accurately describes the contents.

Unlock Deck
Unlock for access to all 197 flashcards in this deck.
Unlock Deck
k this deck
11
Which of the following can be characterized as a strong acid?
A) 0.001 M HCl
B) 3.0 M CH3COOH
C) both a and b
D) neither a nor b
A) 0.001 M HCl
B) 3.0 M CH3COOH
C) both a and b
D) neither a nor b

Unlock Deck
Unlock for access to all 197 flashcards in this deck.
Unlock Deck
k this deck
12
The species H+ can be called which of the following?
A) hydrogen ion
B) proton
C) either a or b
D) neither a nor b
A) hydrogen ion
B) proton
C) either a or b
D) neither a nor b

Unlock Deck
Unlock for access to all 197 flashcards in this deck.
Unlock Deck
k this deck
13
Which of the following occurs when hydrogen chloride gas is dissolved in water to form hydrochloric acid?
A) A complex between HCl and water is formed.
B) H+ is transferred from HCl to water.
C) H+ is transferred from water to HCl.
D) There is no reaction, HCl molecules remain present.
A) A complex between HCl and water is formed.
B) H+ is transferred from HCl to water.
C) H+ is transferred from water to HCl.
D) There is no reaction, HCl molecules remain present.

Unlock Deck
Unlock for access to all 197 flashcards in this deck.
Unlock Deck
k this deck
14
Which of the following can be characterized as a strong base?
A) 0.001 M KOH
B) 3.0 M NH3
C) both a and b
D) neither a nor b
A) 0.001 M KOH
B) 3.0 M NH3
C) both a and b
D) neither a nor b

Unlock Deck
Unlock for access to all 197 flashcards in this deck.
Unlock Deck
k this deck
15
Which of the following can be characterized as a strong base?
A) 0.001 M KOH
B) 3.0 M KOH
C) both a and b
D) neither a nor b
A) 0.001 M KOH
B) 3.0 M KOH
C) both a and b
D) neither a nor b

Unlock Deck
Unlock for access to all 197 flashcards in this deck.
Unlock Deck
k this deck
16
According to Arrhenius which species is associated with all bases?
A) H3O+
B) OH-
C) H2O
D) none of these
A) H3O+
B) OH-
C) H2O
D) none of these

Unlock Deck
Unlock for access to all 197 flashcards in this deck.
Unlock Deck
k this deck
17
Which of the following acids is the acid found in gastric fluid?
A) acetic
B) hydrochloric
C) nitric
D) sulfuric
A) acetic
B) hydrochloric
C) nitric
D) sulfuric

Unlock Deck
Unlock for access to all 197 flashcards in this deck.
Unlock Deck
k this deck
18
Which of the following occurs when NaOH is dissolved in water to form a basic solution?
A) A complex between NaOH and water is formed.
B) NaOH breaks up to form Na+(aq), H+(aq) and O2-(aq).
C) NaOH breaks up to form Na+(aq) and OH-(aq).
D) The water molecule loses an H+.
A) A complex between NaOH and water is formed.
B) NaOH breaks up to form Na+(aq), H+(aq) and O2-(aq).
C) NaOH breaks up to form Na+(aq) and OH-(aq).
D) The water molecule loses an H+.

Unlock Deck
Unlock for access to all 197 flashcards in this deck.
Unlock Deck
k this deck
19
Which of the following occurs when the weak base ammonia, NH3, is dissolved in water?
A) A very small number of ammonia molecules break up.
B) A very small number of ammonia molecules take an H+ from the water.
C) A very small number of molecules of NH4OH are formed.
D) A very small number of water molecules take an H+ from ammonia.
A) A very small number of ammonia molecules break up.
B) A very small number of ammonia molecules take an H+ from the water.
C) A very small number of molecules of NH4OH are formed.
D) A very small number of water molecules take an H+ from ammonia.

Unlock Deck
Unlock for access to all 197 flashcards in this deck.
Unlock Deck
k this deck
20
Which of the following can be characterized as a strong acid?
A) HNO3
B) H2SO4
C) both a and b
D) neither a nor b
A) HNO3
B) H2SO4
C) both a and b
D) neither a nor b

Unlock Deck
Unlock for access to all 197 flashcards in this deck.
Unlock Deck
k this deck
21
Which of the following is the conjugate acid of water?
A) HCl
B) H3O+
C) NH4+
D) OH-
A) HCl
B) H3O+
C) NH4+
D) OH-

Unlock Deck
Unlock for access to all 197 flashcards in this deck.
Unlock Deck
k this deck
22
Which base is an important starting material in the manufacture of fertilizers?
A) LiOH
B) Mg(OH)2
C) NaOH
D) NH3
A) LiOH
B) Mg(OH)2
C) NaOH
D) NH3

Unlock Deck
Unlock for access to all 197 flashcards in this deck.
Unlock Deck
k this deck
23
Which base is sometimes used as a laxative?
A) LiOH
B) Mg(OH)2
C) NaOH
D) NH3
A) LiOH
B) Mg(OH)2
C) NaOH
D) NH3

Unlock Deck
Unlock for access to all 197 flashcards in this deck.
Unlock Deck
k this deck
24
Which of the following is not a base?
A) HPO42-
B) OH-
C) NH3
D) None, they are all bases.
A) HPO42-
B) OH-
C) NH3
D) None, they are all bases.

Unlock Deck
Unlock for access to all 197 flashcards in this deck.
Unlock Deck
k this deck
25
Which of the following bases can accepted only one proton?
A) NH3
B) CH3NH2
C) (CH3)2NH
D) all of them
A) NH3
B) CH3NH2
C) (CH3)2NH
D) all of them

Unlock Deck
Unlock for access to all 197 flashcards in this deck.
Unlock Deck
k this deck
26
Which of the following can be characterized as a strong electrolyte?
A) 0.001 M HCl
B) 3.0 M HCl
C) both a and b
D) neither a nor b
A) 0.001 M HCl
B) 3.0 M HCl
C) both a and b
D) neither a nor b

Unlock Deck
Unlock for access to all 197 flashcards in this deck.
Unlock Deck
k this deck
27
Ions produced from which of the following acids play an important role in biochemistry?
A) acetic
B) boric
C) nitric
D) phosphoric
A) acetic
B) boric
C) nitric
D) phosphoric

Unlock Deck
Unlock for access to all 197 flashcards in this deck.
Unlock Deck
k this deck
28
Which of the following can be characterized as a strong electrolyte?
A) 0.001 M HCl
B) 3.0 M CH3COOH
C) both a and b
D) neither a nor b
A) 0.001 M HCl
B) 3.0 M CH3COOH
C) both a and b
D) neither a nor b

Unlock Deck
Unlock for access to all 197 flashcards in this deck.
Unlock Deck
k this deck
29
Which of the following is not an acid?
A) H3O+
B) H2CO3
C) HPO42-
D) None, they are all acids.
A) H3O+
B) H2CO3
C) HPO42-
D) None, they are all acids.

Unlock Deck
Unlock for access to all 197 flashcards in this deck.
Unlock Deck
k this deck
30
How many protons can be lost by acetic acid, CH3COOH?
A) 1
B) 2
C) 3
D) 4
A) 1
B) 2
C) 3
D) 4

Unlock Deck
Unlock for access to all 197 flashcards in this deck.
Unlock Deck
k this deck
31
Which of the following is the conjugate base of water?
A) H3O+
B) NaOH
C) OH-
D) O2-
A) H3O+
B) NaOH
C) OH-
D) O2-

Unlock Deck
Unlock for access to all 197 flashcards in this deck.
Unlock Deck
k this deck
32
Which of the following can be characterized as a strong electrolyte?
A) 0.001 M KOH
B) 3.0 M KOH
C) both a and b
D) neither a nor b
A) 0.001 M KOH
B) 3.0 M KOH
C) both a and b
D) neither a nor b

Unlock Deck
Unlock for access to all 197 flashcards in this deck.
Unlock Deck
k this deck
33
How many protons can be lost by citric acid, H3C6H5O6?
A) 1
B) 3
C) 5
D) 8
A) 1
B) 3
C) 5
D) 8

Unlock Deck
Unlock for access to all 197 flashcards in this deck.
Unlock Deck
k this deck
34
Which of the following is a diprotic acid?
A) HCl
B) HNO3
C) H2SO4
D) H3PO4
A) HCl
B) HNO3
C) H2SO4
D) H3PO4

Unlock Deck
Unlock for access to all 197 flashcards in this deck.
Unlock Deck
k this deck
35
Which of the following is the Brønsted-Lowry definition of an acid?
A) a hydroxide ion acceptor
B) a proton acceptor
C) a proton donor
D) none of these
A) a hydroxide ion acceptor
B) a proton acceptor
C) a proton donor
D) none of these

Unlock Deck
Unlock for access to all 197 flashcards in this deck.
Unlock Deck
k this deck
36
Which of the following is the conjugate acid of ammonia, NH3?
A) H+
B) H3O+
C) NH2-
D) NH4+
A) H+
B) H3O+
C) NH2-
D) NH4+

Unlock Deck
Unlock for access to all 197 flashcards in this deck.
Unlock Deck
k this deck
37
Which of the following is the conjugate base of acetic acid, CH3COOH?
A) H3O+
B) CH3COOH2+
C) CH3COO-
D) OH-
A) H3O+
B) CH3COOH2+
C) CH3COO-
D) OH-

Unlock Deck
Unlock for access to all 197 flashcards in this deck.
Unlock Deck
k this deck
38
Which of the following is the Brønsted-Lowry definition of a base?
A) a hydroxide ion acceptor
B) a proton acceptor
C) a proton donor
D) none of these
A) a hydroxide ion acceptor
B) a proton acceptor
C) a proton donor
D) none of these

Unlock Deck
Unlock for access to all 197 flashcards in this deck.
Unlock Deck
k this deck
39
Which of the following acids has long been used as a test for proteins?
A) acetic
B) boric
C) nitric
D) sulfuric
A) acetic
B) boric
C) nitric
D) sulfuric

Unlock Deck
Unlock for access to all 197 flashcards in this deck.
Unlock Deck
k this deck
40
Which of the following is a triprotic acid?
A) HCl
B) HNO3
C) H2SO4
D) H3PO4
A) HCl
B) HNO3
C) H2SO4
D) H3PO4

Unlock Deck
Unlock for access to all 197 flashcards in this deck.
Unlock Deck
k this deck
41
Which of the following bases has the weakest conjugate acid?
A) CH3COO-
B) C6H5O-
C) HSO4-
D) H2PO4-
A) CH3COO-
B) C6H5O-
C) HSO4-
D) H2PO4-

Unlock Deck
Unlock for access to all 197 flashcards in this deck.
Unlock Deck
k this deck
42
What is the name of the acid which dissociates to give the nitrate ion, NO3-?
A) hydronitric acid
B) nitric acid
C) nitritic acid
D) nitrous acid
A) hydronitric acid
B) nitric acid
C) nitritic acid
D) nitrous acid

Unlock Deck
Unlock for access to all 197 flashcards in this deck.
Unlock Deck
k this deck
43
Which of the following is the conjugate base of HPO42-?
A) H3PO4
B) H2PO4-
C) PO43-
D) OH-
A) H3PO4
B) H2PO4-
C) PO43-
D) OH-

Unlock Deck
Unlock for access to all 197 flashcards in this deck.
Unlock Deck
k this deck
44
Which of the following is the conjugate base of H2PO4-?
A) H3PO4
B) HPO42-
C) PO43-
D) OH-
A) H3PO4
B) HPO42-
C) PO43-
D) OH-

Unlock Deck
Unlock for access to all 197 flashcards in this deck.
Unlock Deck
k this deck
45
Which of the following species can be characterized as amphiprotic?
A) H2O
B) HCO3-
C) HPO42-
D) all of them
A) H2O
B) HCO3-
C) HPO42-
D) all of them

Unlock Deck
Unlock for access to all 197 flashcards in this deck.
Unlock Deck
k this deck
46
What is the name of the acid which dissociates to give the cyanide ion, CN-?
A) cyanic acid
B) cyanidic acid
C) cyanous
D) hydrocyanic acid
A) cyanic acid
B) cyanidic acid
C) cyanous
D) hydrocyanic acid

Unlock Deck
Unlock for access to all 197 flashcards in this deck.
Unlock Deck
k this deck
47
Which of the following is the conjugate acid of HPO42-
A) H3PO4
B) H2PO4-
C) PO43-
D) H3O+
A) H3PO4
B) H2PO4-
C) PO43-
D) H3O+

Unlock Deck
Unlock for access to all 197 flashcards in this deck.
Unlock Deck
k this deck
48
What is the name of the acid which dissociates to give the nitrite ion, NO2-?
A) hydronitric acid
B) nitric acid
C) nitritic acid
D) nitrous acid
A) hydronitric acid
B) nitric acid
C) nitritic acid
D) nitrous acid

Unlock Deck
Unlock for access to all 197 flashcards in this deck.
Unlock Deck
k this deck
49
Which of the following is the conjugate acid of H2PO4-?
A) H3PO4
B) HPO42-
C) PO43-
D) H3O+
A) H3PO4
B) HPO42-
C) PO43-
D) H3O+

Unlock Deck
Unlock for access to all 197 flashcards in this deck.
Unlock Deck
k this deck
50
Sulfites, salts of the anion, SO32- are found in wines as preservatives. The acid associated with the sulfite ion is H2SO3. What is the name of H2SO3?
A) hydrosulfuric acid
B) hydrosulfuous acid
C) sulfuric acid
D) sulfurous acid
A) hydrosulfuric acid
B) hydrosulfuous acid
C) sulfuric acid
D) sulfurous acid

Unlock Deck
Unlock for access to all 197 flashcards in this deck.
Unlock Deck
k this deck
51
Which of the following statements best describes the acid/base relationship?
A) As acid strengths increase conjugate base strengths increase.
B) As base strengths increase conjugate acid strengths decrease.
C) both a and b
D) neither a nor b
A) As acid strengths increase conjugate base strengths increase.
B) As base strengths increase conjugate acid strengths decrease.
C) both a and b
D) neither a nor b

Unlock Deck
Unlock for access to all 197 flashcards in this deck.
Unlock Deck
k this deck
52
In an acid-base equilibrium the equilibrium lies in the direction which favors which species?
A) the stronger acid
B) the stronger base
C) both a and b
D) neither a nor b
A) the stronger acid
B) the stronger base
C) both a and b
D) neither a nor b

Unlock Deck
Unlock for access to all 197 flashcards in this deck.
Unlock Deck
k this deck
53
Which of the following species is not amphiprotic?
A) C2H3O2-
B) HC3H2O4-
C) HC4H4O4-
D) None, all of them are amphiprotic.
A) C2H3O2-
B) HC3H2O4-
C) HC4H4O4-
D) None, all of them are amphiprotic.

Unlock Deck
Unlock for access to all 197 flashcards in this deck.
Unlock Deck
k this deck
54
Which of the following is the conjugate base of H3PO4?
A) H2PO4-
B) HPO42-
C) PO43-
D) OH-
A) H2PO4-
B) HPO42-
C) PO43-
D) OH-

Unlock Deck
Unlock for access to all 197 flashcards in this deck.
Unlock Deck
k this deck
55
Which of the following is the conjugate acid of PO43-?
A) H3PO4
B) H2PO4-
C) HPO42-
D) H3O+
A) H3PO4
B) H2PO4-
C) HPO42-
D) H3O+

Unlock Deck
Unlock for access to all 197 flashcards in this deck.
Unlock Deck
k this deck
56
Which of the following acids has the strongest conjugate base?
A) CH3COOH
B) C6H5OH
C) H2SO4
D) H3PO4
A) CH3COOH
B) C6H5OH
C) H2SO4
D) H3PO4

Unlock Deck
Unlock for access to all 197 flashcards in this deck.
Unlock Deck
k this deck
57
Which of the following statements best describes the acid/base relationship?
A) As acid strengths decrease conjugate base strengths increase.
B) As base strengths decrease conjugate acid strengths decrease.
C) both a and b
D) neither a and b
A) As acid strengths decrease conjugate base strengths increase.
B) As base strengths decrease conjugate acid strengths decrease.
C) both a and b
D) neither a and b

Unlock Deck
Unlock for access to all 197 flashcards in this deck.
Unlock Deck
k this deck
58
Which of the following acids has the weakest conjugate base?
A) CH3COOH
B) C6H5OH
C) H2SO4
D) H3PO4
A) CH3COOH
B) C6H5OH
C) H2SO4
D) H3PO4

Unlock Deck
Unlock for access to all 197 flashcards in this deck.
Unlock Deck
k this deck
59
Which of the following statements best describes the acid/base relationship?
A) As acid strengths increase conjugate base strengths increase.
B) As base strengths increase conjugate acid strengths increase.
C) both a and b
D) neither a nor b
A) As acid strengths increase conjugate base strengths increase.
B) As base strengths increase conjugate acid strengths increase.
C) both a and b
D) neither a nor b

Unlock Deck
Unlock for access to all 197 flashcards in this deck.
Unlock Deck
k this deck
60
Which of the following bases has the strongest conjugate acid?
A) CH3COO-
B) C6H5O-
C) HSO4-
D) H2PO4-
A) CH3COO-
B) C6H5O-
C) HSO4-
D) H2PO4-

Unlock Deck
Unlock for access to all 197 flashcards in this deck.
Unlock Deck
k this deck
61
When comparing the strength of two weak acids which factor do we use to determine which is the stronger of the two?
A) the formula weights of the acids
B) the number of protons the acids can lose
C) the size of the acid dissociation constants
D) none of the above
A) the formula weights of the acids
B) the number of protons the acids can lose
C) the size of the acid dissociation constants
D) none of the above

Unlock Deck
Unlock for access to all 197 flashcards in this deck.
Unlock Deck
k this deck
62
Hydrocyanic acid, HCN, is a weaker acid than ammonium ion, NH4+. When hydrocyanic acid reacts with ammonia, NH3, what species are present in the solution in the largest amount?
A) hydrocyanic acid, HCN, and ammonia, NH3
B) hydrocyanic acid, HCN, and ammonium ion, NH4+
C) cyanide ion, CN-, and ammonia, NH3
D) cyanide ion, CN-, and ammonium ion, NH4+
A) hydrocyanic acid, HCN, and ammonia, NH3
B) hydrocyanic acid, HCN, and ammonium ion, NH4+
C) cyanide ion, CN-, and ammonia, NH3
D) cyanide ion, CN-, and ammonium ion, NH4+

Unlock Deck
Unlock for access to all 197 flashcards in this deck.
Unlock Deck
k this deck
63
When acetic acid is dissolved in water which of the following is true of the equilibrium which is established as represented below? 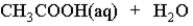

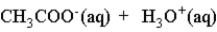
A) It lies very far to the left.
B) It lies slightly to the left.
C) It lies very far to the right.
D) It lies slightly to the right.
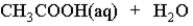

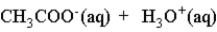
A) It lies very far to the left.
B) It lies slightly to the left.
C) It lies very far to the right.
D) It lies slightly to the right.

Unlock Deck
Unlock for access to all 197 flashcards in this deck.
Unlock Deck
k this deck
64
When comparing two acids which of the following is true?
A) the stronger acid has the larger Ka
B) the stronger acid has the smaller pKa
C) both a and b
D) neither a nor b
A) the stronger acid has the larger Ka
B) the stronger acid has the smaller pKa
C) both a and b
D) neither a nor b

Unlock Deck
Unlock for access to all 197 flashcards in this deck.
Unlock Deck
k this deck
65
Consider the following hypothetical acid-base reaction. A(aq) + B(aq)  C(aq) + D(aq)
C(aq) + D(aq)
Weaker acid weaker base stronger acid stronger base
A) The position of the equilibrium favors the forward reaction.
B) The position of the equilibrium favors the reverse reaction.
C) Neither the forward nor the reverse reaction are favored at equilibrium.
D) The position of equilibrium cannot be predicted from the given data.
 C(aq) + D(aq)
C(aq) + D(aq)Weaker acid weaker base stronger acid stronger base
A) The position of the equilibrium favors the forward reaction.
B) The position of the equilibrium favors the reverse reaction.
C) Neither the forward nor the reverse reaction are favored at equilibrium.
D) The position of equilibrium cannot be predicted from the given data.

Unlock Deck
Unlock for access to all 197 flashcards in this deck.
Unlock Deck
k this deck
66
What are the two acids in the following reaction? NH4+(aq) + HPO42-  NH3 + H2PO4-
NH3 + H2PO4-
A) NH4+ and HPO42-
B) NH4+ and NH3
C) HPO42- and H2PO4-
D) NH4+ and H2PO4-
 NH3 + H2PO4-
NH3 + H2PO4-A) NH4+ and HPO42-
B) NH4+ and NH3
C) HPO42- and H2PO4-
D) NH4+ and H2PO4-

Unlock Deck
Unlock for access to all 197 flashcards in this deck.
Unlock Deck
k this deck
67
When comparing the strength of two weak acids which factor do we use to determine which is the stronger of the two?
A) the formula weights of the acids
B) the number of protons the acids can lose
C) the values of the pKa's of the acids
D) none of the above
A) the formula weights of the acids
B) the number of protons the acids can lose
C) the values of the pKa's of the acids
D) none of the above

Unlock Deck
Unlock for access to all 197 flashcards in this deck.
Unlock Deck
k this deck
68
The pKa of hydrocyanic acid is 9.31. What is the Ka of hydrocyanic acid?
A) 1.3 × 10-24
B) 1.3 × 10-10
C) 4.9 × 10-10
D) 2.0 × 109
A) 1.3 × 10-24
B) 1.3 × 10-10
C) 4.9 × 10-10
D) 2.0 × 109

Unlock Deck
Unlock for access to all 197 flashcards in this deck.
Unlock Deck
k this deck
69
In an acid-base equilibrium the equilibrium lies in the direction which favors which species?
A) the weaker acid
B) the weaker base
C) both a and b
D) neither a nor b
A) the weaker acid
B) the weaker base
C) both a and b
D) neither a nor b

Unlock Deck
Unlock for access to all 197 flashcards in this deck.
Unlock Deck
k this deck
70
Cyanide ion, CN-, is a stronger base than ammonia, NH3. When ammonia reacts with hydrocyanic acid, HCN, what species are present in the solution in the largest amount?
A) hydrocyanic acid, HCN, and ammonia, NH3
B) hydrocyanic acid, HCN, and ammonium ion, NH4+
C) cyanide ion, CN-, and ammonia, NH3
D) cyanide ion, CN-, and ammonium ion, NH4+
A) hydrocyanic acid, HCN, and ammonia, NH3
B) hydrocyanic acid, HCN, and ammonium ion, NH4+
C) cyanide ion, CN-, and ammonia, NH3
D) cyanide ion, CN-, and ammonium ion, NH4+

Unlock Deck
Unlock for access to all 197 flashcards in this deck.
Unlock Deck
k this deck
71
The Ka for lactic acid is 8.4 × 10-4. What is the pKa of lactic acid?
A) -10.92
B) -3.08
C) 3.08
D) 10.92
A) -10.92
B) -3.08
C) 3.08
D) 10.92

Unlock Deck
Unlock for access to all 197 flashcards in this deck.
Unlock Deck
k this deck
72
In an acid-base equilibrium the equilibrium lies in the direction which favors which species?
A) the stronger acid
B) the weaker base
C) both a and b
D) neither a nor b
A) the stronger acid
B) the weaker base
C) both a and b
D) neither a nor b

Unlock Deck
Unlock for access to all 197 flashcards in this deck.
Unlock Deck
k this deck
73
When HCl is dissolved in water which of the following is true of the equilibrium which is established?
A) It lies virtually completely to the left.
B) It lies slightly to the left.
C) It lies virtually completely to the right.
D) It lies slightly to the right.
A) It lies virtually completely to the left.
B) It lies slightly to the left.
C) It lies virtually completely to the right.
D) It lies slightly to the right.

Unlock Deck
Unlock for access to all 197 flashcards in this deck.
Unlock Deck
k this deck
74
Acetic acid, CH3COOH, is a stronger acid than the ammonium ion, NH4+. When acetic acid reacts with ammonia, NH3, what species are present in the solution in the largest amount?
A) acetic acid, CH3COOH and ammonia, NH3
B) acetic acid, CH3COOH, and ammonium, NH4+
C) acetate ion, CH3COO-, and ammonia, NH3
D) acetate ion, CH3COO-, and ammonium ion, NH4+
A) acetic acid, CH3COOH and ammonia, NH3
B) acetic acid, CH3COOH, and ammonium, NH4+
C) acetate ion, CH3COO-, and ammonia, NH3
D) acetate ion, CH3COO-, and ammonium ion, NH4+

Unlock Deck
Unlock for access to all 197 flashcards in this deck.
Unlock Deck
k this deck
75
The Ka for boric acid is 7.3 × 10¯10. What is the pKa of boric acid?
A) -9.14
B) -4.86
C) 4.86
D) 9.14
A) -9.14
B) -4.86
C) 4.86
D) 9.14

Unlock Deck
Unlock for access to all 197 flashcards in this deck.
Unlock Deck
k this deck
76
Ammonia , NH3, is a stronger base than the acetate ion, CH3COO-. When acetic acid, CH3COOH, reacts with ammonia, NH3, what species are present in the solution in the largest amount?
A) acetic acid, CH3COOH, and ammonia, NH3
B) acetic acid, CH3COOH, and ammonium, NH4+
C) acetate ion, CH3COO-, and ammonia, NH3
D) acetate ion, CH3COO-, and ammonium ion, NH4+
A) acetic acid, CH3COOH, and ammonia, NH3
B) acetic acid, CH3COOH, and ammonium, NH4+
C) acetate ion, CH3COO-, and ammonia, NH3
D) acetate ion, CH3COO-, and ammonium ion, NH4+

Unlock Deck
Unlock for access to all 197 flashcards in this deck.
Unlock Deck
k this deck
77
When comparing two acids which of the following is true?
A) the stronger acid has the larger Ka
B) the stronger acid has the larger pKa
C) both a and b
D) neither a nor b
A) the stronger acid has the larger Ka
B) the stronger acid has the larger pKa
C) both a and b
D) neither a nor b

Unlock Deck
Unlock for access to all 197 flashcards in this deck.
Unlock Deck
k this deck
78
When comparing the strength of two weak acids which factor(s) do we use to determine which is the stronger of the two?
A) the size of the acid dissociation constants
B) the values of the pKa's of the acids
C) either a or b
D) neither a nor b
A) the size of the acid dissociation constants
B) the values of the pKa's of the acids
C) either a or b
D) neither a nor b

Unlock Deck
Unlock for access to all 197 flashcards in this deck.
Unlock Deck
k this deck
79
When NH3 is dissolved in water which of the following is true of the equilibrium which is established as represented below? NH3(aq) + H2O(l)  NH4+(aq) + OH-(aq)
NH4+(aq) + OH-(aq)
A) It lies virtually completely to the left.
B) It lies slightly to the left.
C) It lies virtually completely to the right.
D) It lies slightly to the right.
 NH4+(aq) + OH-(aq)
NH4+(aq) + OH-(aq)A) It lies virtually completely to the left.
B) It lies slightly to the left.
C) It lies virtually completely to the right.
D) It lies slightly to the right.

Unlock Deck
Unlock for access to all 197 flashcards in this deck.
Unlock Deck
k this deck
80
When comparing two acids which of the following is true?
A) the stronger acid has the smaller Ka
B) the stronger acid has the larger pKa
C) both a and b
D) neither a nor b
A) the stronger acid has the smaller Ka
B) the stronger acid has the larger pKa
C) both a and b
D) neither a nor b

Unlock Deck
Unlock for access to all 197 flashcards in this deck.
Unlock Deck
k this deck



