Deck 5: Chemical Bonding and Nomenclature
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/230
Play
Full screen (f)
Deck 5: Chemical Bonding and Nomenclature
1
The number of valence electrons in silicon is ________.
A) 2
B) 4
C) 6
D) 8
A) 2
B) 4
C) 6
D) 8
4
2
The number of valence electrons in sulfur is ________.
A) 2
B) 4
C) 6
D) 8
A) 2
B) 4
C) 6
D) 8
6
3
The number of valence electrons in aluminum is ________.
A) 2
B) 3
C) 5
D) 7
A) 2
B) 3
C) 5
D) 7
3
4
The number of valence electrons in magnesium is ________.
A) 2
B) 4
C) 6
D) 8
A) 2
B) 4
C) 6
D) 8

Unlock Deck
Unlock for access to all 230 flashcards in this deck.
Unlock Deck
k this deck
5
Which of the following elements has the lowest number of valence electrons?
A) magnesium
B) lithium
C) argon
D) phosphorus
A) magnesium
B) lithium
C) argon
D) phosphorus

Unlock Deck
Unlock for access to all 230 flashcards in this deck.
Unlock Deck
k this deck
6
The number of valence electrons in nitrogen is ________.
A) 2
B) 3
C) 5
D) 6
A) 2
B) 3
C) 5
D) 6

Unlock Deck
Unlock for access to all 230 flashcards in this deck.
Unlock Deck
k this deck
7
How many covalent bonds can a single oxygen atom form with other elements?
A) 3
B) 4
C) 2
D) 1
A) 3
B) 4
C) 2
D) 1

Unlock Deck
Unlock for access to all 230 flashcards in this deck.
Unlock Deck
k this deck
8
Which phrase best describes a covalent bond between two atoms?
A) a stable arrangement of charged atoms held together by electrostatic forces
B) a stable arrangement of atoms made by sharing two electrons between adjacent atoms
C) uncommon, as electrons are free to jump from one atom to another
D) an exchange of nuclei between two atoms
A) a stable arrangement of charged atoms held together by electrostatic forces
B) a stable arrangement of atoms made by sharing two electrons between adjacent atoms
C) uncommon, as electrons are free to jump from one atom to another
D) an exchange of nuclei between two atoms

Unlock Deck
Unlock for access to all 230 flashcards in this deck.
Unlock Deck
k this deck
9
Which of the following represents the correct Lewis dot structure for selenium (Se)?
A)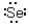
B)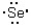
C)
D)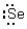
A)
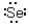
B)
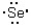
C)

D)
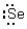

Unlock Deck
Unlock for access to all 230 flashcards in this deck.
Unlock Deck
k this deck
10
Every single COVALENT bond represents ________ electrons.
A) one
B) two
C) four
D) One cannot say unless the atoms that make up the bond are known.
A) one
B) two
C) four
D) One cannot say unless the atoms that make up the bond are known.

Unlock Deck
Unlock for access to all 230 flashcards in this deck.
Unlock Deck
k this deck
11
Which of the following may not exist as a diatomic molecule?
A) oxygen
B) nitrogen
C) argon
D) hydrogen
A) oxygen
B) nitrogen
C) argon
D) hydrogen

Unlock Deck
Unlock for access to all 230 flashcards in this deck.
Unlock Deck
k this deck
12
To follow the octet rule, how many electrons must a neutral sulfur atom gain when forming a negative ion?
A) 1
B) 2
C) 6
D) 8
A) 1
B) 2
C) 6
D) 8

Unlock Deck
Unlock for access to all 230 flashcards in this deck.
Unlock Deck
k this deck
13
Which of the following elements has the highest number of valence electrons?
A) magnesium
B) lithium
C) argon
D) phosphorus
A) magnesium
B) lithium
C) argon
D) phosphorus

Unlock Deck
Unlock for access to all 230 flashcards in this deck.
Unlock Deck
k this deck
14
Which of the following substances is molecular (composed of individual molecules) in composition?
A) carbon in coal
B) drinking water
C) a copper ingot
D) helium in a balloon
A) carbon in coal
B) drinking water
C) a copper ingot
D) helium in a balloon

Unlock Deck
Unlock for access to all 230 flashcards in this deck.
Unlock Deck
k this deck
15
How many valence electrons does a single, neutral iodine atom have?
A) 3
B) 4
C) 5
D) 7
A) 3
B) 4
C) 5
D) 7

Unlock Deck
Unlock for access to all 230 flashcards in this deck.
Unlock Deck
k this deck
16
Which of the following exists as a diatomic molecule?
A) neon
B) oxygen
C) phosphorus
D) silicon
A) neon
B) oxygen
C) phosphorus
D) silicon

Unlock Deck
Unlock for access to all 230 flashcards in this deck.
Unlock Deck
k this deck
17
Which of the following is considered an elemental substance?
A) I₂ (iodine)
B) H₂O (water)
C) Fe (an iron nail)
D) A and C are elemental substances.
E) All of the preceding are elemental substances.
A) I₂ (iodine)
B) H₂O (water)
C) Fe (an iron nail)
D) A and C are elemental substances.
E) All of the preceding are elemental substances.

Unlock Deck
Unlock for access to all 230 flashcards in this deck.
Unlock Deck
k this deck
18
Which of the following statements best describes an ionic bond?
A) a strong lattice of positively and negatively charged atoms held together by electrical forces
B) the bonding type present in bound, non-metal atoms
C) the equal sharing of two electrons between adjacent atoms
D) the bonding type present between two metal atoms
A) a strong lattice of positively and negatively charged atoms held together by electrical forces
B) the bonding type present in bound, non-metal atoms
C) the equal sharing of two electrons between adjacent atoms
D) the bonding type present between two metal atoms

Unlock Deck
Unlock for access to all 230 flashcards in this deck.
Unlock Deck
k this deck
19
The number of valence electrons in neon is ________.
A) 2
B) 4
C) 6
D) 8
A) 2
B) 4
C) 6
D) 8

Unlock Deck
Unlock for access to all 230 flashcards in this deck.
Unlock Deck
k this deck
20
How many covalent bonds can an element of Group VA form with other elements?
A) 4
B) 3
C) 2
D) 1
A) 4
B) 3
C) 2
D) 1

Unlock Deck
Unlock for access to all 230 flashcards in this deck.
Unlock Deck
k this deck
21
Which of the following elements has the least number of valence electrons?
A) krypton
B) potassium
C) calcium
D) aluminum
A) krypton
B) potassium
C) calcium
D) aluminum

Unlock Deck
Unlock for access to all 230 flashcards in this deck.
Unlock Deck
k this deck
22
How many unpaired electrons does the oxide anion (O₂-) have?
A) 3
B) 2
C) 1
D) 0
A) 3
B) 2
C) 1
D) 0

Unlock Deck
Unlock for access to all 230 flashcards in this deck.
Unlock Deck
k this deck
23
The expected formula between potassium and sulfur is ________.
A) KS
B) K₂S3
C) K₂S
D) KS2
A) KS
B) K₂S3
C) K₂S
D) KS2

Unlock Deck
Unlock for access to all 230 flashcards in this deck.
Unlock Deck
k this deck
24
Which of the following pairs has the same number valence electrons?
A) magnesium and potassium
B) lithium and calcium
C) argon and xenon
D) phosphorus and tellurium
A) magnesium and potassium
B) lithium and calcium
C) argon and xenon
D) phosphorus and tellurium

Unlock Deck
Unlock for access to all 230 flashcards in this deck.
Unlock Deck
k this deck
25
What is the most likely formula of the neutral, ionic compound formed by magnesium and oxygen?
A) Mg2O
B) MgO₂
C) Mg3O₂
D) MgO
A) Mg2O
B) MgO₂
C) Mg3O₂
D) MgO

Unlock Deck
Unlock for access to all 230 flashcards in this deck.
Unlock Deck
k this deck
26
The expected formula between sodium and oxygen is ________.
A) NaO
B) Na₂O₂
C) Na₂O
D) Na₂O₃
A) NaO
B) Na₂O₂
C) Na₂O
D) Na₂O₃

Unlock Deck
Unlock for access to all 230 flashcards in this deck.
Unlock Deck
k this deck
27
Methane has ________ covalent bonds and ________ lone pairs.
A) 4; 2
B) 4; 1
C) 4; 0
D) 2; 4
A) 4; 2
B) 4; 1
C) 4; 0
D) 2; 4

Unlock Deck
Unlock for access to all 230 flashcards in this deck.
Unlock Deck
k this deck
28
What is the number of single bonds in C₂H₆O?
A) 5
B) 6
C) 7
D) 8
A) 5
B) 6
C) 7
D) 8

Unlock Deck
Unlock for access to all 230 flashcards in this deck.
Unlock Deck
k this deck
29
The expected formula between aluminum and oxygen is ________.
A) AlO
B) AlO₂
C) AL₂O
D) AL₂O₃
A) AlO
B) AlO₂
C) AL₂O
D) AL₂O₃

Unlock Deck
Unlock for access to all 230 flashcards in this deck.
Unlock Deck
k this deck
30
The expected formula between silicon and fluorine is ________.
A) SiF
B) SiF₂
C) SiF4
D) SiF6
A) SiF
B) SiF₂
C) SiF4
D) SiF6

Unlock Deck
Unlock for access to all 230 flashcards in this deck.
Unlock Deck
k this deck
31
What is the most likely formula of the neutral, ionic compound formed by lithium and nitrogen?
A) LI₂N
B) LI₃N
C) LiN
D) LiN₂
A) LI₂N
B) LI₃N
C) LiN
D) LiN₂

Unlock Deck
Unlock for access to all 230 flashcards in this deck.
Unlock Deck
k this deck
32
The expected formula between calcium and oxygen is ________.
A) CaO
B) CaO₂
C) Ca₂O
D) Ca₂O5
A) CaO
B) CaO₂
C) Ca₂O
D) Ca₂O5

Unlock Deck
Unlock for access to all 230 flashcards in this deck.
Unlock Deck
k this deck
33
The expected formula between magnesium and sulfur is ________.
A) MgS
B) MgS2
C) Mg2S
D) Mg2S3
A) MgS
B) MgS2
C) Mg2S
D) Mg2S3

Unlock Deck
Unlock for access to all 230 flashcards in this deck.
Unlock Deck
k this deck
34
The expected formula between calcium and iodine is ________.
A) CaI
B) CaL₂
C) Ca₂I
D) Ca₂I₃
A) CaI
B) CaL₂
C) Ca₂I
D) Ca₂I₃

Unlock Deck
Unlock for access to all 230 flashcards in this deck.
Unlock Deck
k this deck
35
The expected formula between hydrogen and nitrogen is ________.
A) HN
B) NH₂
C) NH₃
D) NH₄
A) HN
B) NH₂
C) NH₃
D) NH₄

Unlock Deck
Unlock for access to all 230 flashcards in this deck.
Unlock Deck
k this deck
36
The expected binary compound between magnesium and bromine is ________.
A) MgBr
B) MgBr2
C) Mg2Br
D) Mg2Br2
A) MgBr
B) MgBr2
C) Mg2Br
D) Mg2Br2

Unlock Deck
Unlock for access to all 230 flashcards in this deck.
Unlock Deck
k this deck
37
Which of the following has the same number of valence electrons as sulfur?
A) nitrogen
B) oxygen
C) arsenic
D) boron
A) nitrogen
B) oxygen
C) arsenic
D) boron

Unlock Deck
Unlock for access to all 230 flashcards in this deck.
Unlock Deck
k this deck
38
Water has ________ covalent bonds and ________ lone pairs.
A) 2; 2
B) 2; 4
C) 2; 0
D) 2; 6
A) 2; 2
B) 2; 4
C) 2; 0
D) 2; 6

Unlock Deck
Unlock for access to all 230 flashcards in this deck.
Unlock Deck
k this deck
39
Which of the following does not have the same number of valence electrons as the other three elements?
A) nitrogen
B) phosphorus
C) beryllium
D) arsenic
A) nitrogen
B) phosphorus
C) beryllium
D) arsenic

Unlock Deck
Unlock for access to all 230 flashcards in this deck.
Unlock Deck
k this deck
40
How many valence electrons are needed to construct the Lewis dot structure of the BCL₃ molecule?
A) 11
B) 21
C) 24
D) 32
A) 11
B) 21
C) 24
D) 32

Unlock Deck
Unlock for access to all 230 flashcards in this deck.
Unlock Deck
k this deck
41
The phosphate anion has ________ valence electrons.
A) 29
B) 30
C) 32
D) 36
A) 29
B) 30
C) 32
D) 36

Unlock Deck
Unlock for access to all 230 flashcards in this deck.
Unlock Deck
k this deck
42
Nitrogen trifluoride has ________ bonds, ________ of which are single bonds.
A) 3; 3
B) 5; 1
C) 7; 2
D) 8; 3
A) 3; 3
B) 5; 1
C) 7; 2
D) 8; 3

Unlock Deck
Unlock for access to all 230 flashcards in this deck.
Unlock Deck
k this deck
43
The number of valence electrons in the nitrate anion is ________.
A) 23
B) 24
C) 28
D) 30
A) 23
B) 24
C) 28
D) 30

Unlock Deck
Unlock for access to all 230 flashcards in this deck.
Unlock Deck
k this deck
44
Which of the following has at least one double bond?
A) hydrogen
B) carbon dioxide
C) chlorine
D) nitrogen
A) hydrogen
B) carbon dioxide
C) chlorine
D) nitrogen

Unlock Deck
Unlock for access to all 230 flashcards in this deck.
Unlock Deck
k this deck
45
Hydrazine, N₂H₄, has ________ covalent bonds and ________ lone pairs.
A) 2; 2
B) 4; 2
C) 3; 4
D) 5; 2
A) 2; 2
B) 4; 2
C) 3; 4
D) 5; 2

Unlock Deck
Unlock for access to all 230 flashcards in this deck.
Unlock Deck
k this deck
46
The number of valence electrons in the carbonate anion is ________.
A) 20
B) 22
C) 24
D) 26
A) 20
B) 22
C) 24
D) 26

Unlock Deck
Unlock for access to all 230 flashcards in this deck.
Unlock Deck
k this deck
47
Each atom in the oxygen molecule contributes ________ valence electrons for a total of ________ electrons
A) 6; 12
B) 6; 8
C) 8; 6
D) 12; 6
A) 6; 12
B) 6; 8
C) 8; 6
D) 12; 6

Unlock Deck
Unlock for access to all 230 flashcards in this deck.
Unlock Deck
k this deck
48
How many resonance structure could be drawn for the nitrate ion?
A) 0
B) 1
C) 2
D) 3
A) 0
B) 1
C) 2
D) 3

Unlock Deck
Unlock for access to all 230 flashcards in this deck.
Unlock Deck
k this deck
49
Carbon tetrafluoride has ________ total valence electrons.
A) 24
B) 28
C) 32
D) 36
A) 24
B) 28
C) 32
D) 36

Unlock Deck
Unlock for access to all 230 flashcards in this deck.
Unlock Deck
k this deck
50
Nitrogen trifluoride has ________ lone pairs of electrons.
A) 2
B) 5
C) 8
D) 10
A) 2
B) 5
C) 8
D) 10

Unlock Deck
Unlock for access to all 230 flashcards in this deck.
Unlock Deck
k this deck
51
Nitrogen trifluoride has ________ valence electrons that are contributed by the nitrogen atom.
A) 5
B) 8
C) 10
D) 12
A) 5
B) 8
C) 10
D) 12

Unlock Deck
Unlock for access to all 230 flashcards in this deck.
Unlock Deck
k this deck
52
Which of the following gases does not have a triple bond?
A) hydrogen cyanide
B) acetylene, C₂H₂
C) carbon dioxide
D) nitrogen
A) hydrogen cyanide
B) acetylene, C₂H₂
C) carbon dioxide
D) nitrogen

Unlock Deck
Unlock for access to all 230 flashcards in this deck.
Unlock Deck
k this deck
53
The number of valence electrons that belong to the oxygens in the carbonate anion is ________.
A) 15
B) 18
C) 20
D) 24
A) 15
B) 18
C) 20
D) 24

Unlock Deck
Unlock for access to all 230 flashcards in this deck.
Unlock Deck
k this deck
54
Which of the following has a triple bond?
A) water
B) carbon dioxide
C) oxygen
D) nitrogen
A) water
B) carbon dioxide
C) oxygen
D) nitrogen

Unlock Deck
Unlock for access to all 230 flashcards in this deck.
Unlock Deck
k this deck
55
Phosphine, PH₃, has ________ covalent pairs and ________ lone pairs.
A) 3; 0
B) 3; 1
C) 3; 2
D) 2; 0
A) 3; 0
B) 3; 1
C) 3; 2
D) 2; 0

Unlock Deck
Unlock for access to all 230 flashcards in this deck.
Unlock Deck
k this deck
56
Which of the following has more than one double bond?
A) carbon monoxide
B) carbon dioxide
C) oxygen
D) nitrogen
A) carbon monoxide
B) carbon dioxide
C) oxygen
D) nitrogen

Unlock Deck
Unlock for access to all 230 flashcards in this deck.
Unlock Deck
k this deck
57
Hydrazine has ________ valence electrons.
A) 14
B) 12
C) 10
D) 8
A) 14
B) 12
C) 10
D) 8

Unlock Deck
Unlock for access to all 230 flashcards in this deck.
Unlock Deck
k this deck
58
Nitrogen trifluoride has ________ valence electrons.
A) 20
B) 22
C) 24
D) 26
A) 20
B) 22
C) 24
D) 26

Unlock Deck
Unlock for access to all 230 flashcards in this deck.
Unlock Deck
k this deck
59
Acetylene, C₂H₂, has ________.
A) two single bonds
B) two double bonds
C) one triple bond
D) both A and C
A) two single bonds
B) two double bonds
C) one triple bond
D) both A and C

Unlock Deck
Unlock for access to all 230 flashcards in this deck.
Unlock Deck
k this deck
60
Which of the following does not have at least one multiple covalent bond?
A) carbon dioxide
B) ethene, C₂H₄
C) hydrogen cyanide
D) ethane, C₂H₆
A) carbon dioxide
B) ethene, C₂H₄
C) hydrogen cyanide
D) ethane, C₂H₆

Unlock Deck
Unlock for access to all 230 flashcards in this deck.
Unlock Deck
k this deck
61
Consider a bond A-B. If the difference in electronegativity is 3.5, the bond is considered ________.
A) ionic
B) covalent
C) polar covalent
D) hydrogen
A) ionic
B) covalent
C) polar covalent
D) hydrogen

Unlock Deck
Unlock for access to all 230 flashcards in this deck.
Unlock Deck
k this deck
62
Identify the most electronegative element.
A) fluorine
B) oxygen
C) carbon
D) sodium
A) fluorine
B) oxygen
C) carbon
D) sodium

Unlock Deck
Unlock for access to all 230 flashcards in this deck.
Unlock Deck
k this deck
63
Which of the following bonds will have the largest difference in electronegativity?
A) HBr
B) NaF
C) H₂
D) HS-
A) HBr
B) NaF
C) H₂
D) HS-

Unlock Deck
Unlock for access to all 230 flashcards in this deck.
Unlock Deck
k this deck
64
The central atom in sulfuric acid is ________.
A) oxygen
B) sulfur
C) hydrogen
D) none of the above
A) oxygen
B) sulfur
C) hydrogen
D) none of the above

Unlock Deck
Unlock for access to all 230 flashcards in this deck.
Unlock Deck
k this deck
65
Identify the most polar covalent bond.
A) C-C
B) C-H
C) C-F
D) C-Cl
A) C-C
B) C-H
C) C-F
D) C-Cl

Unlock Deck
Unlock for access to all 230 flashcards in this deck.
Unlock Deck
k this deck
66
Identify the least polar covalent bond.
A) C-C
B) C-H
C) C-F
D) C-Cl
A) C-C
B) C-H
C) C-F
D) C-Cl

Unlock Deck
Unlock for access to all 230 flashcards in this deck.
Unlock Deck
k this deck
67
What is the chemical formula of calcium phosphate?
A) CaPO₄
B) Ca₂PO₄
C) Ca3PO₄
D) Ca3(PO₄)2
A) CaPO₄
B) Ca₂PO₄
C) Ca3PO₄
D) Ca3(PO₄)2

Unlock Deck
Unlock for access to all 230 flashcards in this deck.
Unlock Deck
k this deck
68
Consider a bond A-B. If the difference in electronegativity is 0.0, the bond is considered ________.
A) ionic
B) covalent
C) polar covalent
D) hydrogen
A) ionic
B) covalent
C) polar covalent
D) hydrogen

Unlock Deck
Unlock for access to all 230 flashcards in this deck.
Unlock Deck
k this deck
69
Identify the least electronegative element.
A) fluorine
B) oxygen
C) carbon
D) sodium
A) fluorine
B) oxygen
C) carbon
D) sodium

Unlock Deck
Unlock for access to all 230 flashcards in this deck.
Unlock Deck
k this deck
70
If the electrons are shared equally between the two atoms, the bond is called ________.
A) ionic
B) non-polar covalent
C) hydrogen bonding
D) polar covalent
A) ionic
B) non-polar covalent
C) hydrogen bonding
D) polar covalent

Unlock Deck
Unlock for access to all 230 flashcards in this deck.
Unlock Deck
k this deck
71
Which of the following is the molecular formula for ozone?
A) O₃
B) O₃-
C) O₃-2
D) O₂-2
A) O₃
B) O₃-
C) O₃-2
D) O₂-2

Unlock Deck
Unlock for access to all 230 flashcards in this deck.
Unlock Deck
k this deck
72
Which of the following bonds will have the smallest difference in electronegativity?
A) HBr
B) NaF
C) H₂
D) HS-
A) HBr
B) NaF
C) H₂
D) HS-

Unlock Deck
Unlock for access to all 230 flashcards in this deck.
Unlock Deck
k this deck
73
Which of the following compounds has a covalent bond?
A) NaCl
B) KBr
C) LiH
D) H₂
A) NaCl
B) KBr
C) LiH
D) H₂

Unlock Deck
Unlock for access to all 230 flashcards in this deck.
Unlock Deck
k this deck
74
What is the chemical name of the compound whose formula is K₂O?
A) dipotassium oxygen
B) potassium oxide
C) potasside oxygen
D) potassium oxygen
A) dipotassium oxygen
B) potassium oxide
C) potasside oxygen
D) potassium oxygen

Unlock Deck
Unlock for access to all 230 flashcards in this deck.
Unlock Deck
k this deck
75
Consider a bond A-B. If the difference in electronegativity is 1.0, the bond is considered ________.
A) ionic
B) covalent
C) polar covalent
D) hydrogen
A) ionic
B) covalent
C) polar covalent
D) hydrogen

Unlock Deck
Unlock for access to all 230 flashcards in this deck.
Unlock Deck
k this deck
76
In a(n) ________ bond, the electrons are transferred.
A) ionic
B) covalent
C) hydrogen bonding
D) polar covalent
A) ionic
B) covalent
C) hydrogen bonding
D) polar covalent

Unlock Deck
Unlock for access to all 230 flashcards in this deck.
Unlock Deck
k this deck
77
Which of the following phrases best describes the H-O bonds present in water (H₂O)?
A) nonpolar and ionic
B) nonpolar and covalent
C) polar and ionic
D) polar and covalent
A) nonpolar and ionic
B) nonpolar and covalent
C) polar and ionic
D) polar and covalent

Unlock Deck
Unlock for access to all 230 flashcards in this deck.
Unlock Deck
k this deck
78
Identify the ionic bond.
A) C-S
B) Na-F
C) N-O
D) F-F
A) C-S
B) Na-F
C) N-O
D) F-F

Unlock Deck
Unlock for access to all 230 flashcards in this deck.
Unlock Deck
k this deck
79
The numerical rating of an atom's ability to attract the shared electrons is called ________.
A) electronegativity
B) ionization
C) covalency
D) redistribution
A) electronegativity
B) ionization
C) covalency
D) redistribution

Unlock Deck
Unlock for access to all 230 flashcards in this deck.
Unlock Deck
k this deck
80
In a(n) ________ bond, the electrons are shared unequally.
A) ionic
B) non-polar covalent
C) hydrogen bonding
D) polar covalent
A) ionic
B) non-polar covalent
C) hydrogen bonding
D) polar covalent

Unlock Deck
Unlock for access to all 230 flashcards in this deck.
Unlock Deck
k this deck



