Deck 16: Nuclear Chemistry
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/117
Play
Full screen (f)
Deck 16: Nuclear Chemistry
1
The only element whose most stable isotope has n/p < 1 is ________.
A) hydrogen
B) carbon
C) oxygen
D) helium
A) hydrogen
B) carbon
C) oxygen
D) helium
hydrogen
2
Which of the following particles has a mass number of 1?
A) proton
B) neutron
C) helium
D) both A and B
A) proton
B) neutron
C) helium
D) both A and B
both A and B
3
Which of the following particles is a nucleon?
A) proton
B) electron
C) positron
D) both A and B
A) proton
B) electron
C) positron
D) both A and B
proton
4
How long will it take for a 5.0 g tritium sample to decay down to 0.313 g? The half-life of tritium is 12 years.
A) 12 yrs
B) 24 yrs
C) 48 yrs
D) 96 yrs
A) 12 yrs
B) 24 yrs
C) 48 yrs
D) 96 yrs

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
5
Which of the following half-lives is the most stable for an isotope?
A) 0.015 hr
B) 0.92 min
C) 56 s
D) 72 s
A) 0.015 hr
B) 0.92 min
C) 56 s
D) 72 s

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
6
Which of the following does not carry proton(s)?
A) 1H
B) alpha rays
C) deuterium
D) gamma-rays
A) 1H
B) alpha rays
C) deuterium
D) gamma-rays

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
7
An alpha particle may be represented by ________.
A)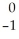 e
e
B) 1n
C) 4He
D) 1H
A)
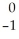 e
eB) 1n
C) 4He
D) 1H

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
8
Which of the following does not carry neutrons?
A) 1n
B) alpha rays
C) 1H
D) deuterium
A) 1n
B) alpha rays
C) 1H
D) deuterium

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
9
Heavy water is ________.
A) water that contains cations such as magnesium, ferric and calcium
B) water whose hydrogens are deuterium
C) water whose hydrogens carry one neutron
D) both B and C
A) water that contains cations such as magnesium, ferric and calcium
B) water whose hydrogens are deuterium
C) water whose hydrogens carry one neutron
D) both B and C

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
10
Which of the following is the easiest to block from penetrating?
A) alpha emissions
B) beta emissions
C) neutrons
D) gamma rays
A) alpha emissions
B) beta emissions
C) neutrons
D) gamma rays

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
11
Which of the following half-lives is the most stable for an isotope?
A) 0.001 s
B) 0.92 s
C) 56 s
D) 72 s
A) 0.001 s
B) 0.92 s
C) 56 s
D) 72 s

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
12
16O, 17O and 18O are ________ of oxygen.
A) isotopes
B) isomers
C) isobars
D) none of the above
A) isotopes
B) isomers
C) isobars
D) none of the above

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
13
Which of the following has an atomic number of 0?
A) proton
B) neutron
C) positron
D) deuterium
A) proton
B) neutron
C) positron
D) deuterium

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
14
Isotopes have ________.
A) the same number of protons but different number of neutrons
B) the same number of neutrons but different number of protons
C) the same number protons and the same number of neutrons
D) different number protons and neutrons
A) the same number of protons but different number of neutrons
B) the same number of neutrons but different number of protons
C) the same number protons and the same number of neutrons
D) different number protons and neutrons

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
15
Mass defect is ________.
A) the amount of mass that is converted to energy during a nuclear reaction
B) the amount of mass produced during a nuclear reaction
C) the amount of energy released when an atom forms from its subatomic particles
D) the amount of energy absorbed when an atom forms from its subatomic particles
A) the amount of mass that is converted to energy during a nuclear reaction
B) the amount of mass produced during a nuclear reaction
C) the amount of energy released when an atom forms from its subatomic particles
D) the amount of energy absorbed when an atom forms from its subatomic particles

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
16
Which of the following does not have an atomic number of 1?
A) proton
B) neutron
C) electron
D) deuterium
A) proton
B) neutron
C) electron
D) deuterium

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
17
An beta particle may be represented by ________.
A)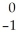 e
e
B) 1n
C) 4He
D) 1H
A)
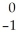 e
eB) 1n
C) 4He
D) 1H

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
18
If the half-life of an isotope is 5 hr, the amount of 20.0 g remaining after 15 hr will be ________.
A) 2.5 g
B) 5.0 g
C) 6.67 g
D) 10.0 g
A) 2.5 g
B) 5.0 g
C) 6.67 g
D) 10.0 g

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
19
Binding energy is ________.
A) the amount of mass that is converted to energy during a nuclear reaction
B) the amount of mass produced during a nuclear reaction
C) the amount of energy released when an atom forms from its subatomic particles
D) the amount of energy absorbed when an atom forms from its subatomic particles
A) the amount of mass that is converted to energy during a nuclear reaction
B) the amount of mass produced during a nuclear reaction
C) the amount of energy released when an atom forms from its subatomic particles
D) the amount of energy absorbed when an atom forms from its subatomic particles

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
20
The n/p ratio is greater than 1 for stable isotopes, when the atomic number is larger than ________.
A) 10
B) 18
C) 20
D) 24
A) 10
B) 18
C) 20
D) 24

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
21
Which is the lightest among the following particles?
A) proton
B) neutron
C) alpha particle
D) beta particle
A) proton
B) neutron
C) alpha particle
D) beta particle

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
22
When nickel-63 is converted to copper-63 ________.
A) an electron is captured
B) an alpha particle is emitted
C) an electron is released
D) a neutron is released
A) an electron is captured
B) an alpha particle is emitted
C) an electron is released
D) a neutron is released

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
23
When a neutron is transformed into a proton what else is emitted?
A) a beta particle
B) a gamma particle
C) an alpha particle
D) None of the above.
A) a beta particle
B) a gamma particle
C) an alpha particle
D) None of the above.

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
24
Identify X in the reaction below. 238U + 12C → 244Cf + X
A) 6 electrons
B) 1 alpha particle
C) 3 protons
D) 6 neutrons
A) 6 electrons
B) 1 alpha particle
C) 3 protons
D) 6 neutrons

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
25
Which of the following particles is the heaviest?
A) deuterium
B) neutron
C) alpha particle
D) beta particle
A) deuterium
B) neutron
C) alpha particle
D) beta particle

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
26
When an unstable nucleus emits a beta particle ________.
A) its mass number is decreased by four units
B) its atomic number is decreased by two units
C) its atomic number is increased by one unit
D) its mass number is decreased by one unit
A) its mass number is decreased by four units
B) its atomic number is decreased by two units
C) its atomic number is increased by one unit
D) its mass number is decreased by one unit

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
27
When an unstable nucleus expels a positron ________.
A) its mass number is decreased by four units
B) its atomic number is decreased by one unit
C) its atomic number is increased by one unit
D) its mass number is decreased by one unit
A) its mass number is decreased by four units
B) its atomic number is decreased by one unit
C) its atomic number is increased by one unit
D) its mass number is decreased by one unit

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
28
Identify X in the reaction below. 27Al + 4He → X + 1n
A) 31P
B) 30P
C) 30S
D) 31Si
A) 31P
B) 30P
C) 30S
D) 31Si

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
29
Which of the following is able to penetrate the easiest through human tissue?
A) alpha rays
B) beta rays
C) neutrons
D) gamma rays
A) alpha rays
B) beta rays
C) neutrons
D) gamma rays

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
30
What is the missing particle in the reaction below? ? + 6Li → 2 4He (alpha particles)
A) 3H
B) 1H
C) 2H
D) 4He
A) 3H
B) 1H
C) 2H
D) 4He

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
31
An gamma particle may be represented by ________.
A)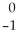 e
e
B)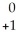 e
e
C)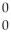 γ
γ
D)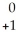 γ
γ
A)
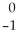 e
eB)
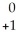 e
eC)
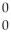 γ
γD)
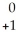 γ
γ
Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
32
Which of the following is not a beta emitting reaction?
A) 231Th → 231Pa
B) 131I → 131Xe
C) 90Sr → 90Y
D) 226Ra → 222Rn
A) 231Th → 231Pa
B) 131I → 131Xe
C) 90Sr → 90Y
D) 226Ra → 222Rn

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
33
The process below is an example of ________. 238U → 234Th + 4He
A) alpha emission
B) beta emission
C) gamma emission
D) neutron emission
A) alpha emission
B) beta emission
C) gamma emission
D) neutron emission

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
34
The mercury-197 to gold-197 conversion is accompanied by ________.
A) an electron capture
B) an electron release
C) an alpha emission
D) a neutron capture
A) an electron capture
B) an electron release
C) an alpha emission
D) a neutron capture

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
35
When an unstable nucleus emits an alpha particle ________.
A) its mass number is decreased by four units
B) its atomic number is decreased by one unit
C) its atomic number is increased by one unit
D) its mass number is decreased by one unit
A) its mass number is decreased by four units
B) its atomic number is decreased by one unit
C) its atomic number is increased by one unit
D) its mass number is decreased by one unit

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
36
Which of the following transformations is not an alpha emission?
A) 14C → 14N
B) 225Ac → 221Fr
C) 241Am → 237Np
D) 208Po → 204Pb
A) 14C → 14N
B) 225Ac → 221Fr
C) 241Am → 237Np
D) 208Po → 204Pb

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
37
When radium-226 is converted to radon-222 ________.
A) an electron is captured
B) an alpha particle is emitted
C) an electron is released
D) a neutron is released
A) an electron is captured
B) an alpha particle is emitted
C) an electron is released
D) a neutron is released

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
38
What is the missing particle in the reaction below? 1p + 0e →?
A) 2p
B) 1H
C) 1n
D) 4He
A) 2p
B) 1H
C) 1n
D) 4He

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
39
Which of the following transformations is not an electron capture?
A) 201Hg → 201Au
B) 81Rb → 81Kr
C) 231Th → 231Pa
D) p → n
A) 201Hg → 201Au
B) 81Rb → 81Kr
C) 231Th → 231Pa
D) p → n

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
40
When carbon-14 is converted to nitrogen-14 ________.
A) an electron is captured
B) an alpha particle is emitted
C) an electron is released
D) a neutron is released
A) an electron is captured
B) an alpha particle is emitted
C) an electron is released
D) a neutron is released

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
41
40Ar, 40K and 40Ca are isotopes.

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
42
The daughter isotope of 203Po when it undergoes alpha-emission is 207Pb.

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
43
An electron is an example of a nucleon.

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
44
The half-life of an isotope decreases as the initial concentration decreases.

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
45
The atomic number of a neutron is 1.

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
46
35Cl and 37Cl are isotopes.

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
47
Both the proton and neutron have the same mass number.

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
48
The n/p > 1 ratio appears in elements whose atomic number is greater than 24.

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
49
A beta particle has a -1 charge.

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
50
Identify X in the reaction below. X + 1n → 142Ba + 91Kr + 3 1n
A) 235Th
B) 238U
C) 235U
D) 234Pa
A) 235Th
B) 238U
C) 235U
D) 234Pa

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
51
The reaction below is an example of ________. 12C + 12C → 24Mg
A) fission
B) fusion
C) isotope coupling
D) transmutation
A) fission
B) fusion
C) isotope coupling
D) transmutation

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
52
A nuclear reaction involves much higher energies than a chemical reaction.

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
53
The larger the half-life value, the more stable the isotope.

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
54
12C is the most stable carbon isotope.

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
55
The main problem that nuclear reactors have when uranium-235 serves as the fuel is ________.
A) the production of more neutrons that the reaction consumes
B) the production of gamma rays
C) the consumption of more alpha particles than the available alpha particles
D) the immense amount of energy produced
A) the production of more neutrons that the reaction consumes
B) the production of gamma rays
C) the consumption of more alpha particles than the available alpha particles
D) the immense amount of energy produced

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
56
Nuclear radiation is harmful because of ________.
A) the high energy particles produced
B) the electromagnetic radiation emitted by a nucleus during the nuclear change
C) the ionizing of biological molecules
D) all of the above
A) the high energy particles produced
B) the electromagnetic radiation emitted by a nucleus during the nuclear change
C) the ionizing of biological molecules
D) all of the above

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
57
The reaction below is an example of ________. 230U → 139Ba + 89Kr + 2 1n
A) fission
B) fusion
C) isotope
D) transmutation
A) fission
B) fusion
C) isotope
D) transmutation

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
58
An alpha particle is neutral.

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
59
Isotopes with the lowest possible n/p ratio are usually the most stable.

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
60
The daughter isotope of 200Hg when it undergoes alpha-emission is 196Pt.

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
61
Uranium-238 can capture a neutron inside a nuclear reactor (a so-called breeder reactor), producing an isotope of plutonium and two beta particles. The plutonium can then itself be used as a nuclear fuel. Write a balanced equation for the breeder reactor's production of plutonium (include isotope number) from uranium-238.

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
62
During the MRI procedure the human is placed in a magnetic field and radio waves are used to probe certain nuclei in the body.

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
63
Calculate the binding energy of oxygen-16 in J/mol. This isotope has an atomic mass of 15.9949 g/mol.

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
64
What is the mass defect of the carbon-14 isotope (in g/mol)? Carbon-14 has an atomic mass of 14.0032 g/mol.

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
65
For the following nuclear reactions, supply the missing isotope, or elementary particle.
14N + 4He → ________ + 1H
14N + 4He → ________ + 1H

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
66
The half life of 14C is 5,715 years.

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
67
For the following nuclear reactions, supply the missing isotope, or elementary particle.
22Na → ________ + +1e
22Na → ________ + +1e

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
68
Fusion reactors of the future hope to make use of the following reaction:
2H + 3H → 4He + 1n
How much energy is released in this process (in J/mol)?
(2H = 2.0141 g/mol; 3H = 3.0160 g/mol; 4He = 4.0026 g/mol; 1n = 1.00866 g/mol)
2H + 3H → 4He + 1n
How much energy is released in this process (in J/mol)?
(2H = 2.0141 g/mol; 3H = 3.0160 g/mol; 4He = 4.0026 g/mol; 1n = 1.00866 g/mol)

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
69
The daughter isotope of 210Po when it undergoes beta-emission is 210At.

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
70
Calculate the binding energy of neon-22 in J/mol. This isotope has an atomic mass of 21.9914 g/mol.

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
71
13C is the carbon isotope used for radioactive carbon dating.

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
72
A fusion reaction creates more energy than a fission reaction.

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
73
Nuclear radiation can cause biological damage by the weakening or breaking of bonds.

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
74
What is the mass defect of the lithium-9 isotope in g/mol? Lithium-9 has an atomic mass of 9.0273 g/mol.

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
75
The half-life of chlorine-34 is 1.52 seconds. How much time will pass before a given sample of chlorine-34 decays by 60%?

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
76
For the following nuclear reactions, supply the missing isotope, or elementary particle.
________ → 226Rn + 4He
________ → 226Rn + 4He

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
77
If nuclear energy is released all at once, a nuclear bomb can be created.

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
78
Many radioactive nuclei release energy in the form of electromagnetic radiation, such as gamma rays.

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
79
A fission reaction is always endothermic.

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
80
Calculate the amount of energy released when this nuclear reaction occurs inside an atomic warhead:
1n + 235U → 137Te + 97Zr + 2 1n
(235U = 235.0439 g/mol; 137Te = 136.9254 g/mol; 97Zr = 96.9110 g/mol; 1n = 1.00866 g/mol)
1n + 235U → 137Te + 97Zr + 2 1n
(235U = 235.0439 g/mol; 137Te = 136.9254 g/mol; 97Zr = 96.9110 g/mol; 1n = 1.00866 g/mol)

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck



