Deck 24: Carbohydrates
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/122
Play
Full screen (f)
Deck 24: Carbohydrates
1
Which of the following is a ketopentose? 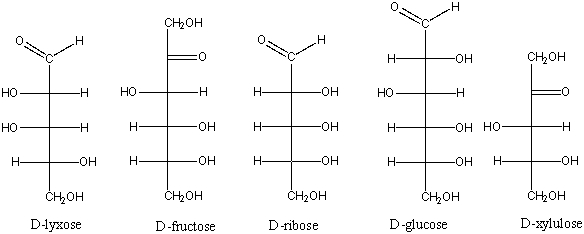
A) D-lyxose
B) D-fructose
C) D-ribose
D) D-glucose
E) D-xylulose

A) D-lyxose
B) D-fructose
C) D-ribose
D) D-glucose
E) D-xylulose
D-xylulose
2
Which of the following best describes the relationship between D-glucose and D-fructose?
A) enantiomers
B) epimers
C) anomers
D) constitutional isomers
E) diastereomers
A) enantiomers
B) epimers
C) anomers
D) constitutional isomers
E) diastereomers
constitutional isomers
3
A monosaccharide with six carbon atoms and an aldehyde functional group is called a _____.
A) hexose
B) ketohexose
C) aldohexose
D) ketoheptose
E) ketose
A) hexose
B) ketohexose
C) aldohexose
D) ketoheptose
E) ketose
aldohexose
4
Which of the following is an aldohexose? 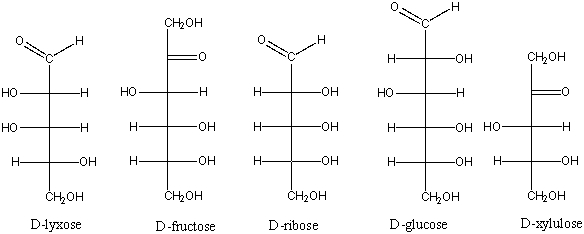
A) D-lyxose
B) D-fructose
C) D-ribose
D) D-glucose
E) D-xylulose

A) D-lyxose
B) D-fructose
C) D-ribose
D) D-glucose
E) D-xylulose

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
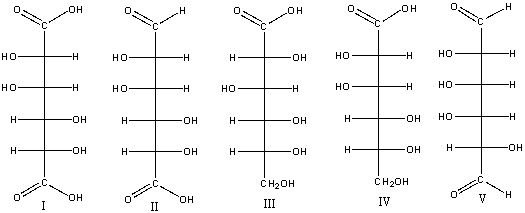
5
Which of the following is (are) L-ketopentose(s)? 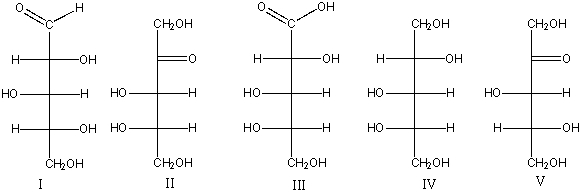
A) I
B) II
C) III
D) IV
E) III & IV

A) I
B) II
C) III
D) IV
E) III & IV

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
6
Which of the following best describes L-galactose?
A) aldopentose
B) aldohexose
C) ketopentose
D) ketohexose
E) aldotetrose
A) aldopentose
B) aldohexose
C) ketopentose
D) ketohexose
E) aldotetrose

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
7
Degradation of synthetic glucose produces _________that is ________.
A) glyceraldehyde, dextrorotatory
B) glyceraldehyde, levorotatory
C) glyceraldehyde, both dextro and levorotatory
D) none of these
A) glyceraldehyde, dextrorotatory
B) glyceraldehyde, levorotatory
C) glyceraldehyde, both dextro and levorotatory
D) none of these

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
8
Which of the following is a ketohexose? 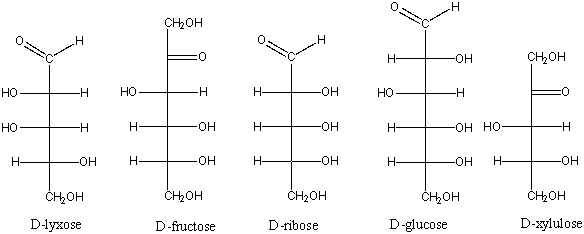
A) D-lyxose
B) D-fructose
C) D-ribose
D) D-glucose
E) D-xylulose

A) D-lyxose
B) D-fructose
C) D-ribose
D) D-glucose
E) D-xylulose

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
9
A monosaccharide with five carbon atoms and a ketone functional group is called a _____.
A) aldose
B) ketohexose
C) aldopentose
D) ketopentose
E) pentose
A) aldose
B) ketohexose
C) aldopentose
D) ketopentose
E) pentose

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
10
Which of the following compounds are a pair of enantiomers? 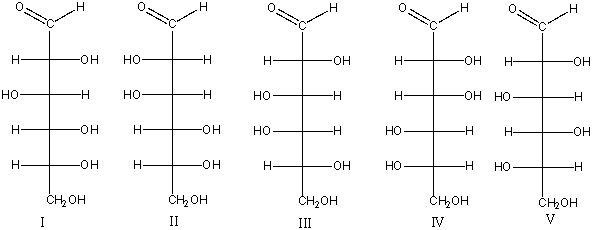
A) I & III
B) II & IV
C) III & V
D) IV
E) none of these

A) I & III
B) II & IV
C) III & V
D) IV
E) none of these

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
11
Polysaccharides are made by joining ________together.
A) disaccharides
B) trisaccharides
C) polysaccharides
D) monosaccharides
E) all of these
A) disaccharides
B) trisaccharides
C) polysaccharides
D) monosaccharides
E) all of these

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
12
Which of the following is (are) L-aldohexose(s)? 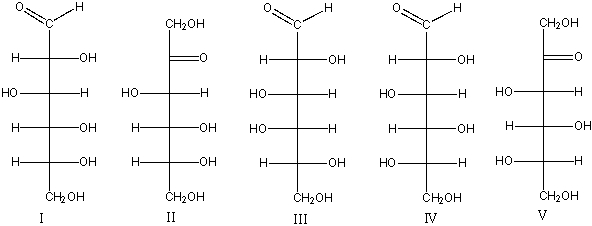
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
13
Simple sugars are called_______ , a term that derives from Latin word for sugar, saccharum.
A) monosaccharides
B) disaccharides
C) polysaccharides
D) saccharides
E) all of these
A) monosaccharides
B) disaccharides
C) polysaccharides
D) saccharides
E) all of these

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
14
In the Fischer projection of D-glyceraldehyde the OH group connected to the chirality center farthest from the carbonyl group is pointing _______.
A) to the left
B) to the right
C) up
D) down
E) all of these
A) to the left
B) to the right
C) up
D) down
E) all of these

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
15
Of the possible stereoisomers for galactose, how many are L-isomers?
A) 8
B) 6
C) 10
D) 3
E) 4
A) 8
B) 6
C) 10
D) 3
E) 4

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
16
Which of the following aldohexoses is (are) dextrorotatory?
A) D-glucose
B) D-galactose
C) D-gulose
D) D-mannose
E) cannot predict
A) D-glucose
B) D-galactose
C) D-gulose
D) D-mannose
E) cannot predict

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
17
Degradation of naturally occurring glucose produces _________that is ________.
A) D-glyceraldehyde, dextrorotatory
B) D-glyceraldehyde, levorotatory
C) L-glyceraldehyde, dextrorotatory
D) L-glyceraldehyde, levorotatory
E) none of these
A) D-glyceraldehyde, dextrorotatory
B) D-glyceraldehyde, levorotatory
C) L-glyceraldehyde, dextrorotatory
D) L-glyceraldehyde, levorotatory
E) none of these

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
18
Plants convert carbon dioxide and water into glucose in presence of sunlight via ________.
A) hydrolysis
B) retrosynthesis
C) Killiani synthesis
D) photosynthesis
E) none of these
A) hydrolysis
B) retrosynthesis
C) Killiani synthesis
D) photosynthesis
E) none of these

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
19
Which of the following is (are) D-aldopentose(s)? 
A) I
B) II
C) III
D) IV
E) I & V

A) I
B) II
C) III
D) IV
E) I & V

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
20
Which of the following is an aldopentose? 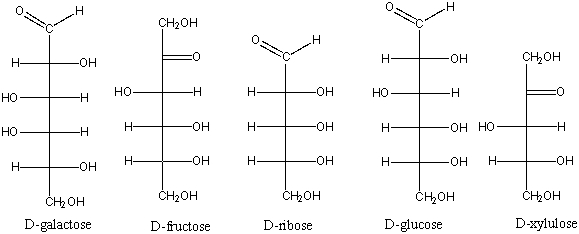
A) D-galactose
B) D-fructose
C) D-ribose
D) D-glucose
E) D-xylulose

A) D-galactose
B) D-fructose
C) D-ribose
D) D-glucose
E) D-xylulose

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
21
Draw a Fisher projection for D-fructose.

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
22
Of the possible stereoisomers for glucose, how many are D-isomers?
A) 8
B) 6
C) 10
D) 3
E) 4
A) 8
B) 6
C) 10
D) 3
E) 4

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
23
Draw the Fisher projection for L-glucose.

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
24
Predict the major product for the following reaction. 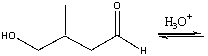
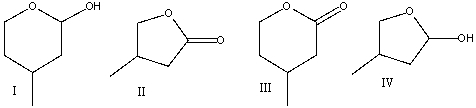
A) I
B) II
C) III
D) IV
E) none of these


A) I
B) II
C) III
D) IV
E) none of these

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
25
Draw the Fisher projection for D-galactose.

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
26
Which carbon in the following monosaccharide is the anomeric carbon? 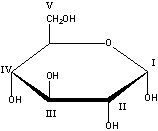
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
27
Provide the reactant(s) for the following reaction. 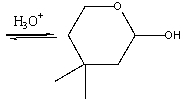


Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
28
How many stereoisomers are possible for glucose?
A) 8
B) 16
C) 10
D) 12
E) 4
A) 8
B) 16
C) 10
D) 12
E) 4

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
29
Of the possible stereoisomers for fructose, how many are D-isomers?
A) 8
B) 6
C) 10
D) 2
E) 4
A) 8
B) 6
C) 10
D) 2
E) 4

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
30
Predict the major product for the following reaction. 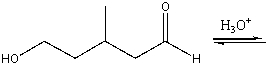
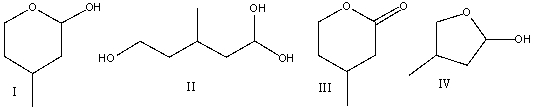
A) I
B) II
C) III
D) IV
E) none of these


A) I
B) II
C) III
D) IV
E) none of these

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
31
Which one of the following is the correct structure for L-fructose? 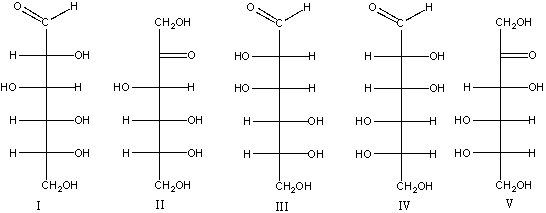
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
32
How many stereoisomers are possible for fructose?
A) 8
B) 6
C) 10
D) 3
E) 4
A) 8
B) 6
C) 10
D) 3
E) 4

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
33
Which one of the following is the correct stereochemical configuration for D-xylose? 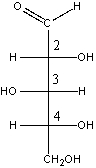
A) 2S,3R,4S
B) 2R,3S,4R
C) 2R,3R,4S
D) 2S,3S,4R
E) 2S,3S,4S

A) 2S,3R,4S
B) 2R,3S,4R
C) 2R,3R,4S
D) 2S,3S,4R
E) 2S,3S,4S

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
34
Which one of the following is the correct stereochemical configuration for D-mannose? 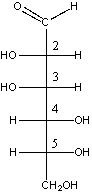
A) 2S,3S,4R,5R
B) 2R,3S,4R,5S
C) 2R,3R,4S,5S
D) 2S,3S,4R,5S
E) 2S,3R,4S,5R

A) 2S,3S,4R,5R
B) 2R,3S,4R,5S
C) 2R,3R,4S,5S
D) 2S,3S,4R,5S
E) 2S,3R,4S,5R

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
35
Draw a Fisher projection for the enantiomer of D-glucose.

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
36
Which of the following is(are) L-hexose(s)? 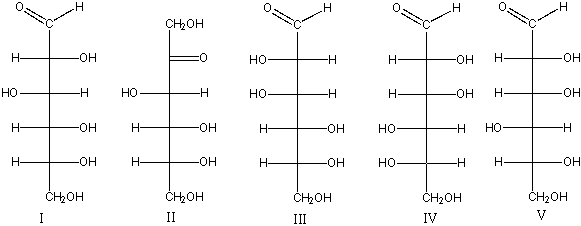
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
37
Which of the following best describes the relationship between -D-glucopyranose and -D-glucopyranose?
A) enantiomers
B) anomers
C) epimers
D) constitutional isomers
E) none of these
A) enantiomers
B) anomers
C) epimers
D) constitutional isomers
E) none of these

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
38
Which one of the following is the correct Fischer projection for D-gulose? 
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
39
Anomers of D-glucopyranose differ in stereochemistry at ____carbon.
A) C1
B) C2
C) C3
D) C4
E) C5
A) C1
B) C2
C) C3
D) C4
E) C5

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
40
Which of the following compounds is(are) an epimer(s) of D-glucose? 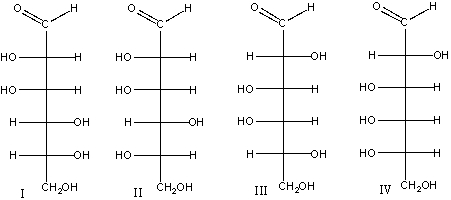
A) I
B) II
C) I & III
D) II &IV
E) I & II

A) I
B) II
C) I & III
D) II &IV
E) I & II

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
41
What is the correct name for the following compound? 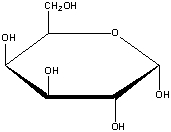
A) -D-glucopyranose
B) -D-allopyranose
C) -D-glucopyranose
D) -D-galactopyranose
E) -D-gulopyranose

A) -D-glucopyranose
B) -D-allopyranose
C) -D-glucopyranose
D) -D-galactopyranose
E) -D-gulopyranose

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
42
What is the correct name for the following compound? 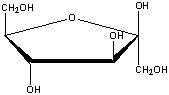
A) -D-fructofuranose
B) -D-sorbofuranose
C) -D-glucopyranose
D) -D- fructofuranose
E) -D-gulopyranose

A) -D-fructofuranose
B) -D-sorbofuranose
C) -D-glucopyranose
D) -D- fructofuranose
E) -D-gulopyranose

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
43
Which of the following compound(s) would NOT undergo mutarotation in aqueous solution? 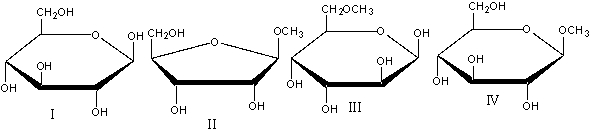
A)I
B)II&IV
C)III
D)IV
E) II, III & IV

A)I
B)II&IV
C)III
D)IV
E) II, III & IV

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
44
Draw the Haworth projection for -D-erythrofuranose.

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
45
Provide the reagents necessary to carry out the following conversion. 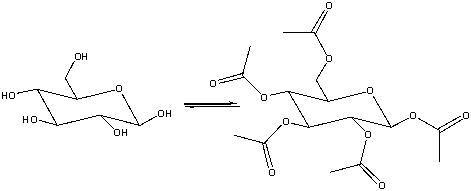


Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
46
Which of the following compound(s) is a glycoside? 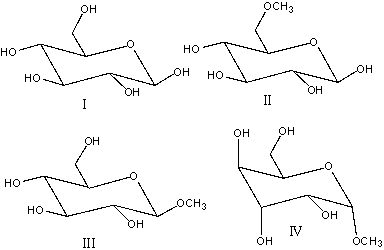
A)I
B)II
C)III
D)I &III & IV
E) II, III & IV

A)I
B)II
C)III
D)I &III & IV
E) II, III & IV

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
47
What is the correct Haworth projection for -D-glucopyranose? 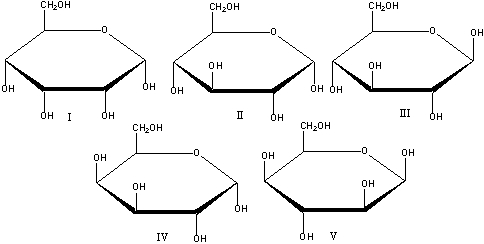
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
48
Which of the following structures represent -D-glucopyranose? 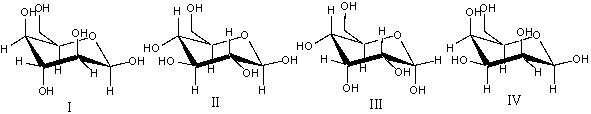
A)I
B)II
C) III
D)IV
E) none of these

A)I
B)II
C) III
D)IV
E) none of these

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
49
Draw the Fischer projection for the open-chain form of the following cyclic monosaccharide. 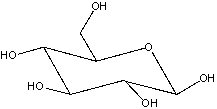


Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
50
Furanose forms of monosaccharides are_____.
A)5-membered cyclic hemiacetals
B)6-membered cyclic acetals
C)5-membered cyclic acetals
D)6-membered cyclic hemiacetals
E) none of these
A)5-membered cyclic hemiacetals
B)6-membered cyclic acetals
C)5-membered cyclic acetals
D)6-membered cyclic hemiacetals
E) none of these

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
51
Pyranose forms of monosaccharides are_____.
A)5-membered cyclic hemiacetals
B)6-membered cyclic acetals
C)5-membered cyclic acetals
D)6-membered cyclic hemiacetals
E) none of these
A)5-membered cyclic hemiacetals
B)6-membered cyclic acetals
C)5-membered cyclic acetals
D)6-membered cyclic hemiacetals
E) none of these

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
52
Draw the chair conformation of -D-galactopyranose.

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
53
Provide the reagents necessary to carry out the following conversion. 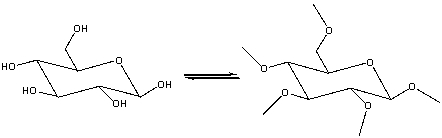


Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
54
What is the correct Haworth projection for -D-allopyranose? 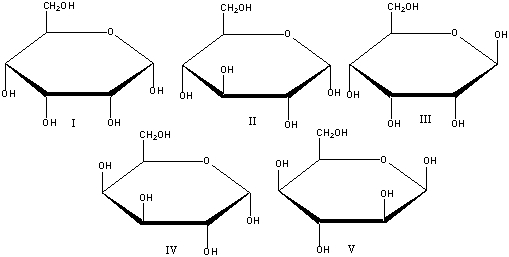
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
55
Draw the chair conformation for the following monosaccharide. 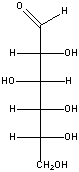


Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
56
Predict the product(s) when -D-galactopyranose reacts with excess acetic anhydride in the presence of pyridine.

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
57
Six-membered cyclic hemiacetal carbohydrates are called ________.
A) furanose
B) pyranose
C) ketopentose
D) aldopentose
E) none of these
A) furanose
B) pyranose
C) ketopentose
D) aldopentose
E) none of these

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
58
Which of the following compound(s) would undergo mutarotation in aqueous solution? 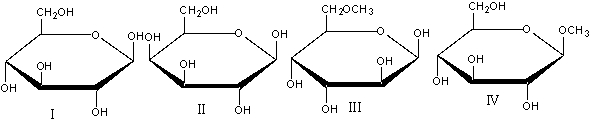
A)I
B)II&III
C)III&IV
D)I&II&III
E) all of these

A)I
B)II&III
C)III&IV
D)I&II&III
E) all of these

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
59
What is the correct name for the following compound? 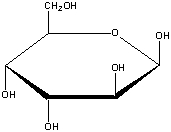
A) -D-idopyranose
B) -D-altopyranose
C) -D-glucopyranose
D) -D-galactopyranose
E) -D-gulopyranose

A) -D-idopyranose
B) -D-altopyranose
C) -D-glucopyranose
D) -D-galactopyranose
E) -D-gulopyranose

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
60
Five-membered cyclic hemiacetal carbohydrates are called ________.
A) furanose
B) pyranose
C) ketohexose
D) aldohexose
E) none of these
A) furanose
B) pyranose
C) ketohexose
D) aldohexose
E) none of these

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
61
Which of the following D-aldoses will produce an optically inactive product when treated with NaBH4/H2O? 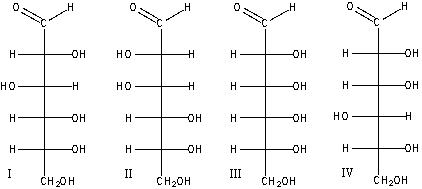
A) I
B) II
C) III
D) IV
E) none of these

A) I
B) II
C) III
D) IV
E) none of these

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
62
Predict the product(s) for the following reaction. 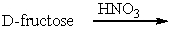
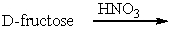

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
63
Predict the product(s) for the following reaction. 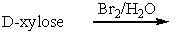
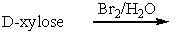

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
64
Predict the product(s) for the following reaction. 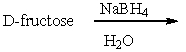
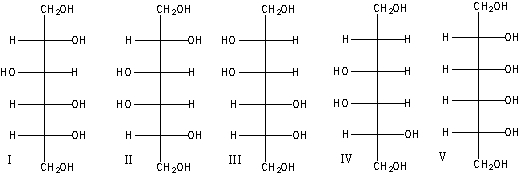
A) I
B) II
C) I & III
D) IV
E) I &V
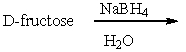

A) I
B) II
C) I & III
D) IV
E) I &V

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
65
Which one of the following compounds would give a positive test with Benedict's solution? 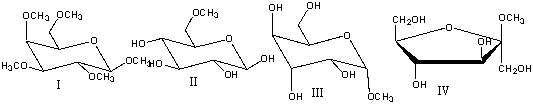
A) I
B) II
C) III
D) IV
E) none of these

A) I
B) II
C) III
D) IV
E) none of these

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
66
D-glucose & D-galactose are ______ epimers of each other.
A) C-1
B) C-2
C) C-3
D) C-4
E) C-5
A) C-1
B) C-2
C) C-3
D) C-4
E) C-5

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
67
When D-threose is treated with NaBH4/H2O, it forms _______.
A) a racemic mixture of alditols
B) a meso alditol
C) an optically active alditol
D) an optically active aldonic acid
E) none of these
A) a racemic mixture of alditols
B) a meso alditol
C) an optically active alditol
D) an optically active aldonic acid
E) none of these

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
68
Predict the product(s) for the following reaction. 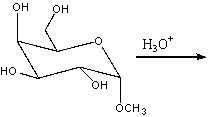
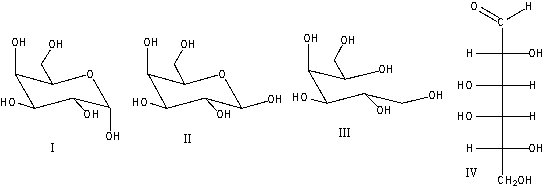
A)I
B)I&II
C)III&IV
D) IV
E) I, II & IV


A)I
B)I&II
C)III&IV
D) IV
E) I, II & IV

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
69
Predict the product(s) for the following reaction. 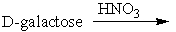
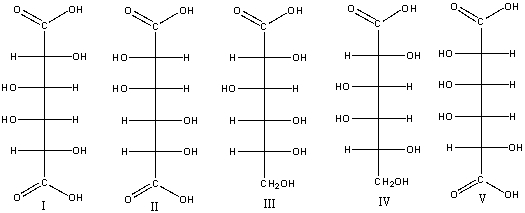
A) I
B) II
C) III
D) IV
E) V
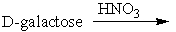

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
70
Which of the following compound(s) would give a positive Tollens test? 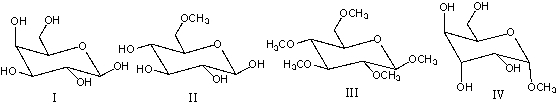
A) I
B) II
C) III
D) IV
E) I & II

A) I
B) II
C) III
D) IV
E) I & II

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
71
When D-ribose is treated with nitric acid, it forms
A) a racemic mixture of aldonic acids
B) a meso aldaric acid
C) an optically active aldaric acid
D) an optically active aldonic acid
E) a meso aldonic acid
A) a racemic mixture of aldonic acids
B) a meso aldaric acid
C) an optically active aldaric acid
D) an optically active aldonic acid
E) a meso aldonic acid

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
72
D-ribulose & D-xylulose are ______epimers of each other.
A) C-1
B) C-2
C) C-3
D) C-4
E) C-5
A) C-1
B) C-2
C) C-3
D) C-4
E) C-5

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
73
Which one of the following compounds is a non-reducing sugar? 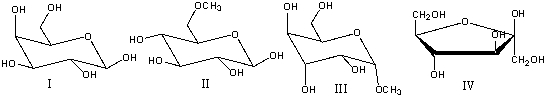
A) I
B) II
C) III
D) IV
E) none of these

A) I
B) II
C) III
D) IV
E) none of these

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
74
Predict the product(s) for the following reaction. 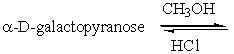
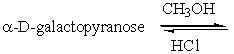

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
75
Which of the following best describes the relationship between D-glucose and D-galactose?
A) enantiomers
B) anomers
C) epimers
D) constitutional isomers
E) none of these
A) enantiomers
B) anomers
C) epimers
D) constitutional isomers
E) none of these

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
76
Which of the following compound(s) would produce an optically active aldaric acid? 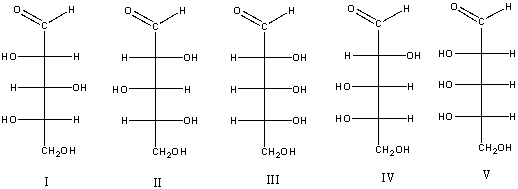
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
77
Predict the product(s) for the following reaction. 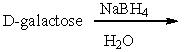
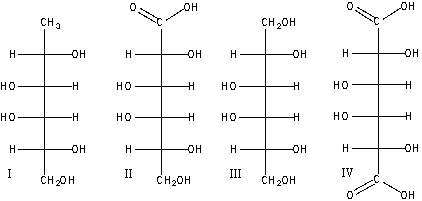
A) I
B) II
C) III
D) IV
E) II & IV
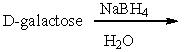

A) I
B) II
C) III
D) IV
E) II & IV

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
78
Predict the product(s) for the following reaction. 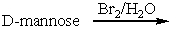
A) I
B) II
C) III
D) IV
E) V
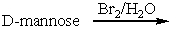

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
79
When D-glucose is treated with aqueous NaOH it undergoes_______.
A) mutarotation
B) oxidation
C) glycoside formation
D) epimerization
E) none of these
A) mutarotation
B) oxidation
C) glycoside formation
D) epimerization
E) none of these

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
80
Provide the reagents necessary to carry out the following conversion. 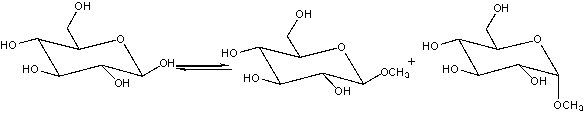


Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck



