Deck 22: Alpha Carbon Chemistry: Enols and Enolates
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/131
Play
Full screen (f)
Deck 22: Alpha Carbon Chemistry: Enols and Enolates
1
Which of the following statements explains the ketone-enol equilibrium shown below? 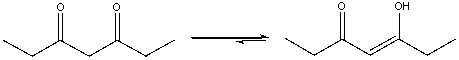
A) simple enols are more stable
B) the enol tautomer is stabilized by the conjugated system
C) the enol tautomer is stabilized by the intramolecular hydrogen bonding
D) the diketone is less coplanar
E) both B & C

A) simple enols are more stable
B) the enol tautomer is stabilized by the conjugated system
C) the enol tautomer is stabilized by the intramolecular hydrogen bonding
D) the diketone is less coplanar
E) both B & C
both B & C
2
Which of the following bases will completely convert 1,4-cyclohexanedione into an enolate?
A) sodium hydroxide
B) sodium ethoxide
C) LDA
D) sodium hydride
E) both C & D
A) sodium hydroxide
B) sodium ethoxide
C) LDA
D) sodium hydride
E) both C & D
both C & D
3
Which one of the following compounds is most likely to favor the enol tautomer over the ketone tautomer? 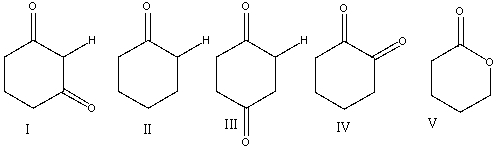
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V
I
4
Which is the most acidic hydrogen in the following compound? 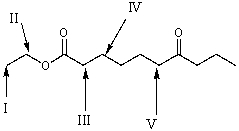
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
5
Provide the structure of the enol when 3,3,6-trimethyl-4-heptanone is treated with acid.

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
6
Which is the most acidic hydrogen in the following compound? 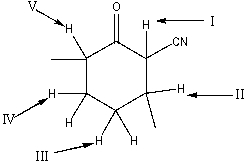
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
7
Which of the following is (are) a keto-enol tautomeric pair(s)? 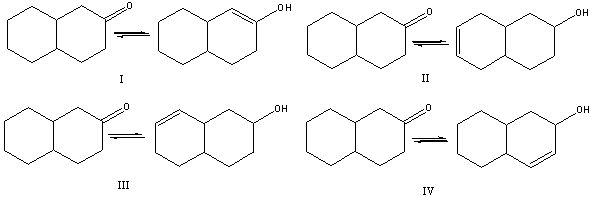
A) I
B) II
C) III
D) IV
E) None of these

A) I
B) II
C) III
D) IV
E) None of these

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
8
Arrange the following indicated hydrogens in decreasing order (most to least) of acidity. 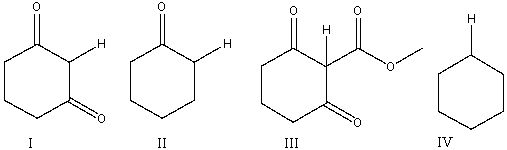
A) I>II>III>IV
B) IV>II>III>I
C) III>II>I>IV
D) II>I>III>IV
E) III>I>II>IV

A) I>II>III>IV
B) IV>II>III>I
C) III>II>I>IV
D) II>I>III>IV
E) III>I>II>IV

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
9
Provide the structure of the enolate when acetophenone is treated with strong base.

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
10
Which of the following compounds represents an enolate? 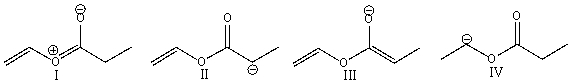
A) I
B) II
C) III
D) IV
E) both B & C

A) I
B) II
C) III
D) IV
E) both B & C

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
11
Which one of the following compounds is most likely to favor the enol tautomer over the ketone tautomer? 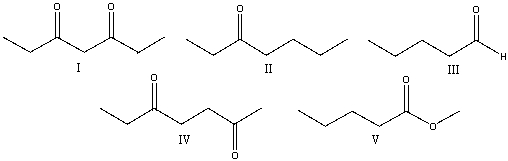
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
12
Which of the following indicated - hydrogens is most acidic? 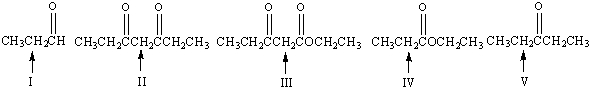
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
13
Explain why the following equilibrium favors the enol tautomer. 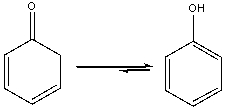


Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
14
Which one of the following compounds is most acidic?
A) ethyl acetoacetate
B) 2-butanone
C) ethyl pentanoate
D) 1-butanol
E) 3-pentanone
A) ethyl acetoacetate
B) 2-butanone
C) ethyl pentanoate
D) 1-butanol
E) 3-pentanone

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
15
Which is the most acidic hydrogen in the following compound? 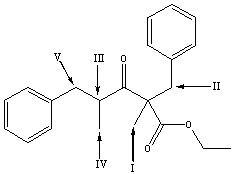
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
16
Which of the following is (are) a keto-enol tautomeric pair(s)? 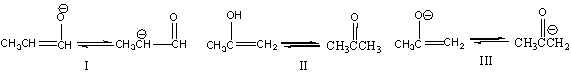
A) I
B) II
C) III
D) I & II
E) I & III
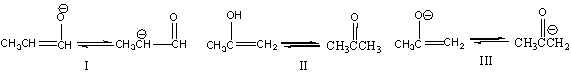
A) I
B) II
C) III
D) I & II
E) I & III

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
17
Which is the most acidic hydrogen in the following compound? 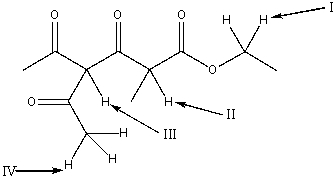
A) I
B) II
C) III
D) IV
E) all of these

A) I
B) II
C) III
D) IV
E) all of these

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
18
Which of the following is (are) a keto-enol tautomeric pair(s)? 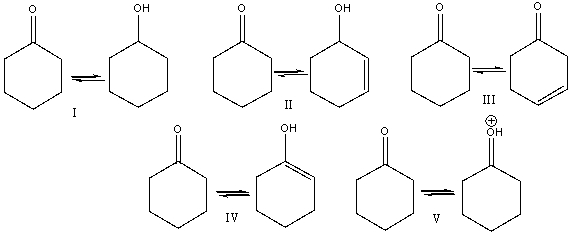
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
19
Which one of the following indicated hydrogens is most acidic? 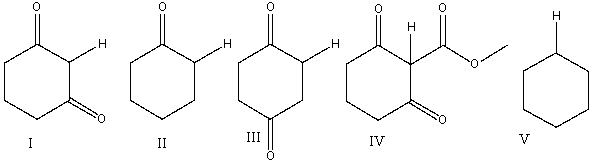
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
20
Explain why the following equilibrium favors the enol tautomer. 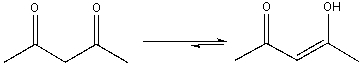


Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
21
Predict the major product for the following reaction. 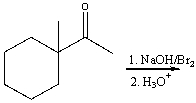
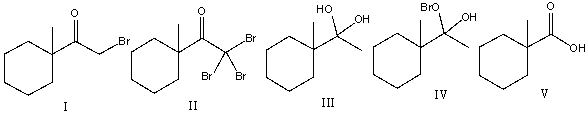
A) I
B) II
C) III
D) IV
E) V


A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
22
Predict the major product for the following reaction. 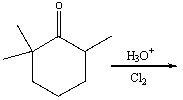
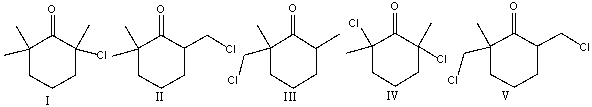
A) I
B) II
C) III
D) IV
E) V


A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
23
Predict the major product for the following reaction. 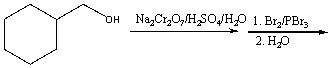
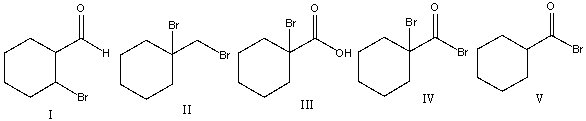
A) I
B) II
C) III
D) IV
E) V
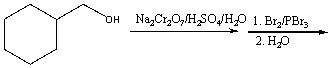

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
24
Which of the following ketones will give a positive iodoform test?
A) 3-heptanone
B) 3-hexanone
C) cyclohexanone
D) 2-pentanone
E) 2-methyl-3-hexanone
A) 3-heptanone
B) 3-hexanone
C) cyclohexanone
D) 2-pentanone
E) 2-methyl-3-hexanone

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
25
Predict the major product for the following reaction. 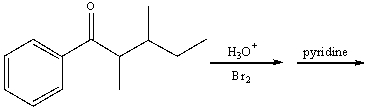
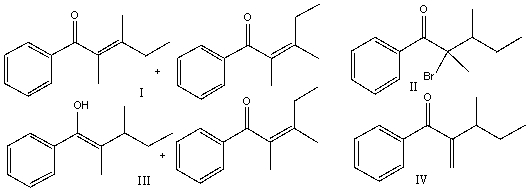
A) I
B) II
C) III
D) IV
E) none of these


A) I
B) II
C) III
D) IV
E) none of these

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
26
Predict the major product for the following reaction. 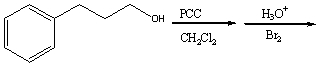
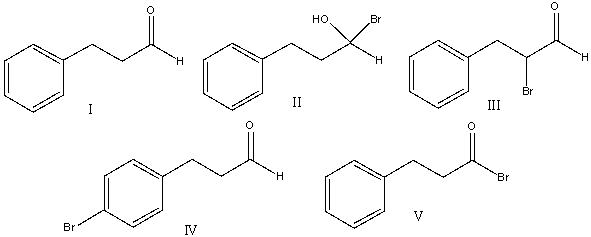
A) I
B) II
C) III
D) IV
E) V


A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
27
Provide the reagents necessary to carry out the following conversion. 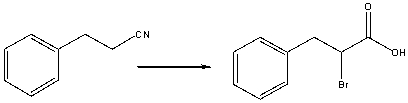


Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
28
Which of the following compounds would undergo racemization in presence of a base? 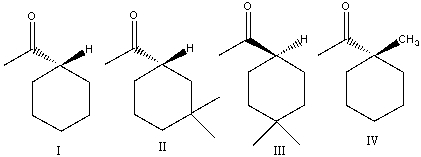
A) I
B) II
C) III
D) IV
E) II & III

A) I
B) II
C) III
D) IV
E) II & III

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
29
Provide both resonance structures of the enolate formed when the following ketone is treated with a base. 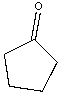


Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
30
Provide the reagents necessary to carry out the following conversion. 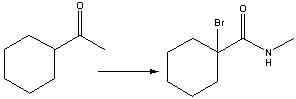


Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
31
Provide the reagents necessary to carry out the following conversion. 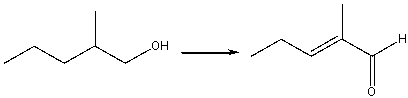


Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
32
Predict the major product for the following reaction sequence. 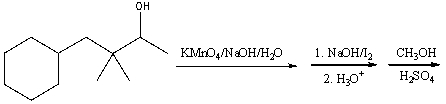
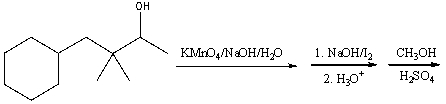

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
33
Predict the major product for the following reaction. 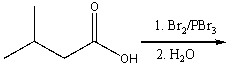
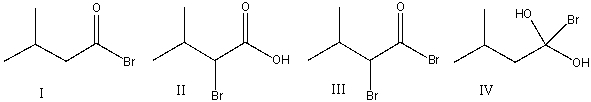
A) I
B) II
C) III
D) IV
E) none of these


A) I
B) II
C) III
D) IV
E) none of these

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
34
Predict the major product for the following reaction. 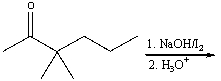
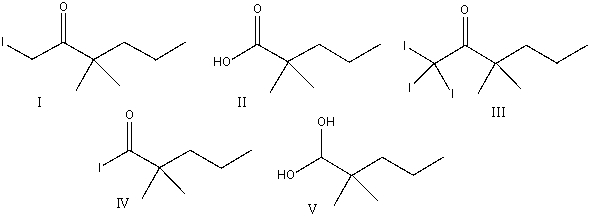
A) I
B) II
C) III
D) IV
E) V


A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
35
Which one of the following compounds would undergo racemization at the -stereocenter in presence of a base? 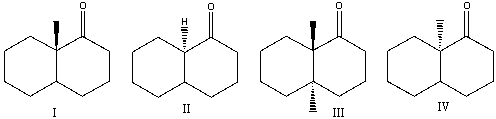
A) I
B) II
C) III
D) IV
E) none of these

A) I
B) II
C) III
D) IV
E) none of these

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
36
Provide both resonance structures of the enolate formed when the following ester is treated with a base. 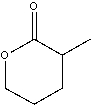


Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
37
Provide both resonance structures of the enolate formed when the following ketone is treated with a base. 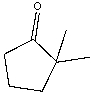


Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
38
Provide the reactant that would yield m-chlorobenzoic acid using the haloform reaction. 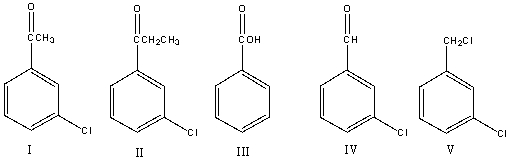
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
39
Provide the reagents necessary to carry out the following conversion. 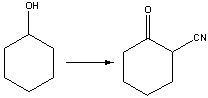


Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
40
Predict the major product for the following reaction and provide a curved arrow mechanism for the formation of the product. 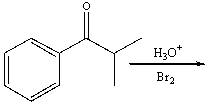


Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
41
Provide the reactant(s) that would give the following possible aldol condensation product. 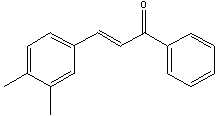


Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
42
Provide the reactants that would give the following aldol condensation product. 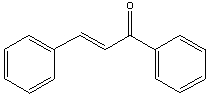
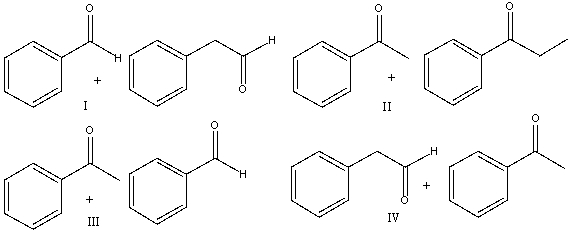
A) I
B) II
C) III
D) IV
E) none of these


A) I
B) II
C) III
D) IV
E) none of these

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
43
Provide the reactant(s) that would give the following aldol condensation product. 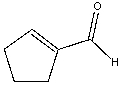
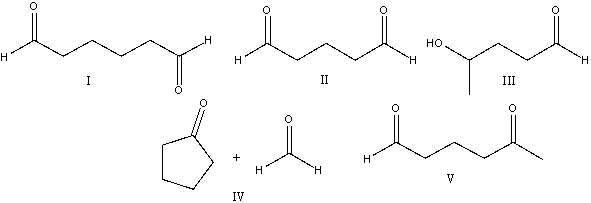
A) I
B) II
C) III
D) IV
E) V


A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
44
Predict the major product for the following reaction. 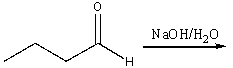
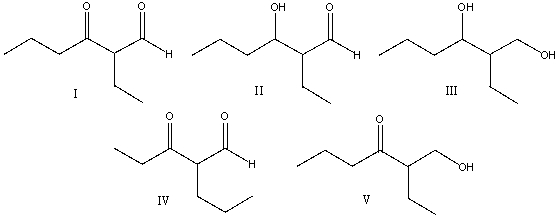
A) I
B) II
C) III
D) IV
E) V


A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
45
Which one of the following compounds does not undergo an aldol addition reaction in the presence of aqueous sodium hydroxide?
A) butanal
B) 2-methylbutanal
C) 3-methylpentanal
D) 2,2-dimethylbutanal
E) none of these
A) butanal
B) 2-methylbutanal
C) 3-methylpentanal
D) 2,2-dimethylbutanal
E) none of these

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
46
Predict the product for the following reaction. 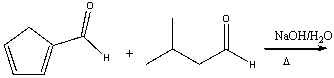


Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
47
Predict the major product for the following reaction. 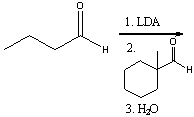
A) I
B) II
C) III
D) IV
E) V


A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
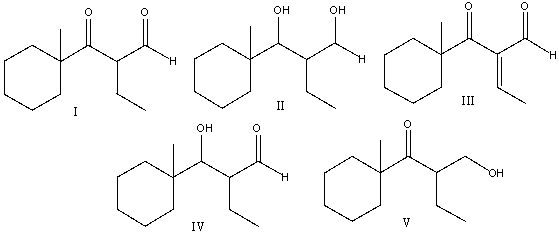
48
Predict the major product(s) for the following reaction. 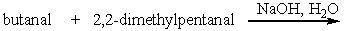
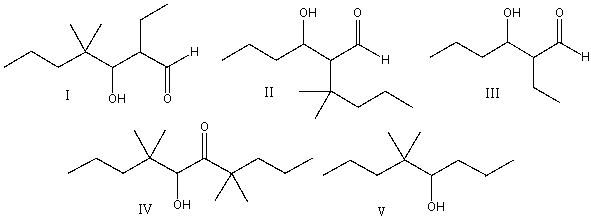
A) I
B) II & IV
C) III
D) I & III
E) V
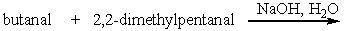

A) I
B) II & IV
C) III
D) I & III
E) V

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
49
Which one of the following is not a possible product when a crossed aldol addition reaction is carried out with ethanal and butanal as reactants? 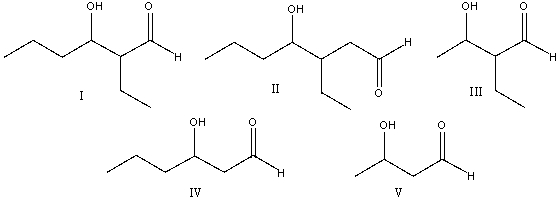
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
50
Predict the product when cyclohexanone reacts with aqueous sodium hydroxide at 100 C. 
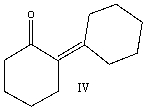
A) I
B) II
C) III
D) IV
E) none of these


A) I
B) II
C) III
D) IV
E) none of these

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
51
Predict the major product for the following reaction. 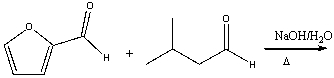
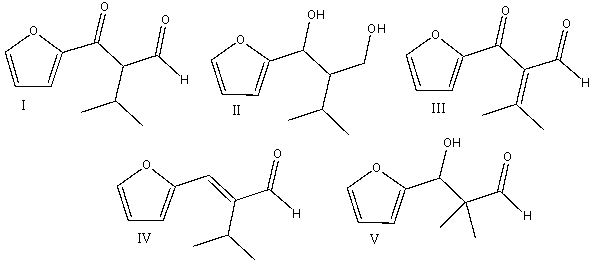
A) I
B) II
C) III
D) IV
E) V


A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
52
Predict the product for the following reaction. 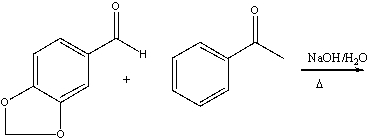
A) I
B) II
C) III
D) IV
E) none of these


A) I
B) II
C) III
D) IV
E) none of these

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
53
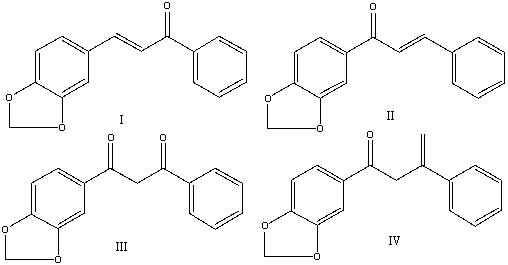
Provide the reactants that would give the following aldol condensation product. 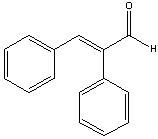
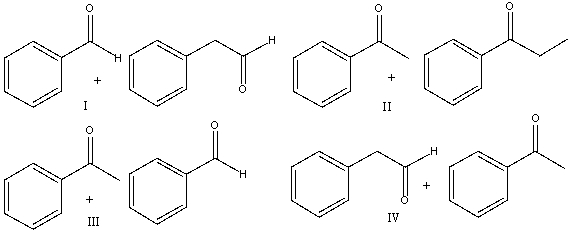
A) I
B) II
C) III
D) IV
E) none of these


A) I
B) II
C) III
D) IV
E) none of these

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
54
Predict the major product for the following reaction. 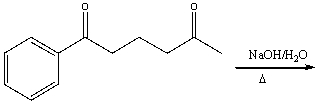
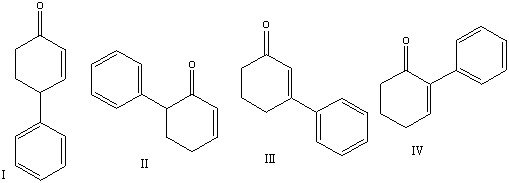
A) I
B) II
C) III
D) IV
E) none of these


A) I
B) II
C) III
D) IV
E) none of these

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
55
Provide the reactant(s) that would give the following aldol condensation product. 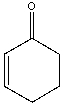
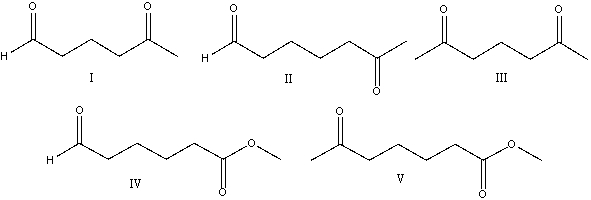
A) I
B) II
C) III
D) IV
E) V


A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
56
Predict the product for the following reaction. 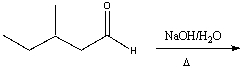
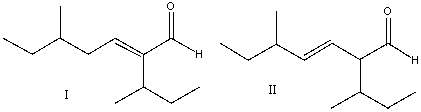
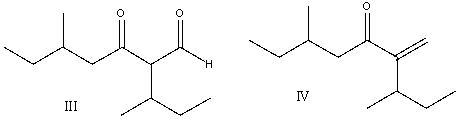
A) I
B) II
C) III
D) IV
E) none of these



A) I
B) II
C) III
D) IV
E) none of these

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
57
Predict the product for the following reaction. 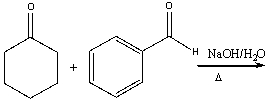
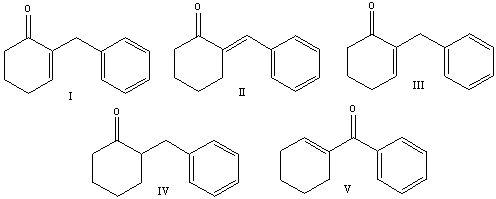
A) I
B) II
C) III
D) IV
E) V


A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
58
Provide the reactant(s) that would give the following possible aldol condensation product. 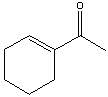
A) I
B) II
C) III
D) IV
E) V


A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
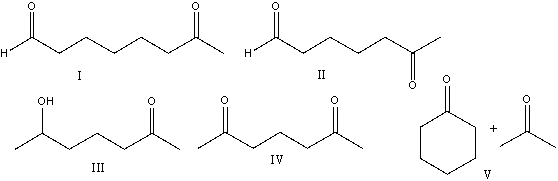
59
Provide the reactants that would give the following aldol condensation product. 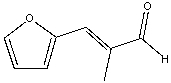


Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
60
Predict the product for the following reaction. 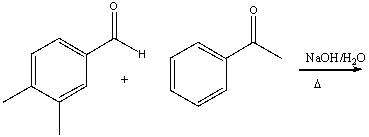


Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
61
Predict the product(s) for the following reaction. 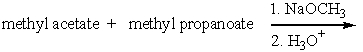

A)I
B)II
C)III
D)IV
E) all of these
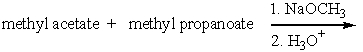

A)I
B)II
C)III
D)IV
E) all of these

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
62
Provide the structure of reactant (X) for the following reaction. 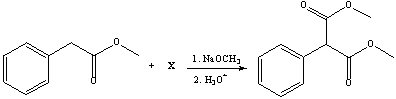
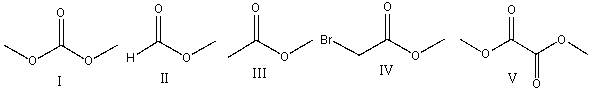
A) I
B) II
C) III
D) IV
E) V
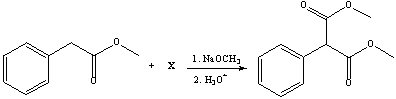

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
63
Provide the reactants that will yield the following compound as crossed Claisen condensation product. 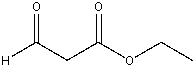


Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
64
Predict the major product for the following reaction. 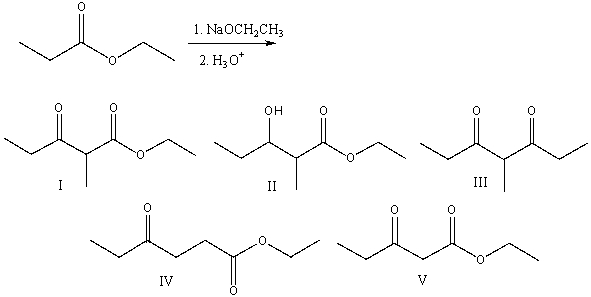
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
65
Predict the product(s) for the following reaction. 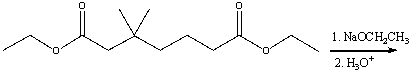
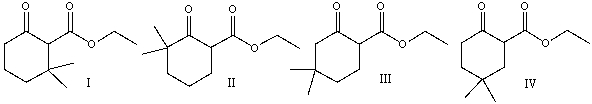
A)I
B)II
C)III
D). I&IV
E) I & III


A)I
B)II
C)III
D). I&IV
E) I & III

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
66
Provide the reactant(s) that will yield the following Claisen condensation product. 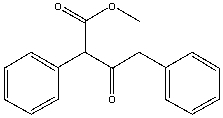


Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
67
Explain why CH3ONa should not be used for Claisen condensation of ethylbutanoate.

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
68
Predict the product for the following Dieckmann-like cyclization. 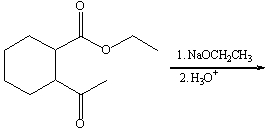
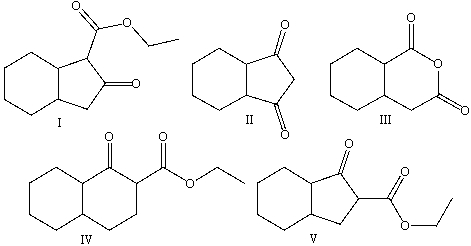
A) I
B) II
C) III
D) IV
E) V


A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
69
Which of the following compounds can be prepared using the Dieckmann condensation? 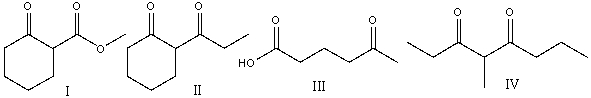
A)I
B)II
C)III
D)IV
E) II & IV

A)I
B)II
C)III
D)IV
E) II & IV

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
70
Predict the major product for the following reaction sequence. 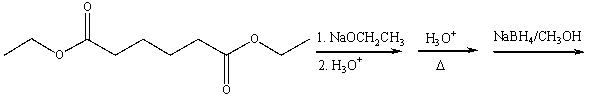
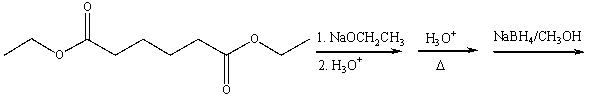

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
71
Provide a stepwise synthesis for the following compound starting with cycloheptene and using the Dieckmann cyclization. 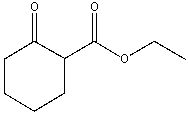


Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
72
Which one of the following is a major product in a Claisen condensation?
A) -keto ester
B) -keto ester
C) -hydroxy ester
D) -hydroxyester
E) -diketone
A) -keto ester
B) -keto ester
C) -hydroxy ester
D) -hydroxyester
E) -diketone

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
73
Predict the product(s) for the following reaction. 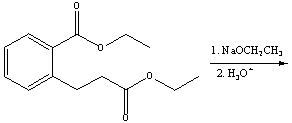
A)I
B)II
C)III
D)IV
E) V


A)I
B)II
C)III
D)IV
E) V

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
74
Predict the major product for the following reaction. 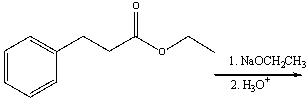


Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
75
Predict the product(s) for the following reaction. 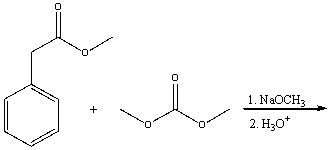
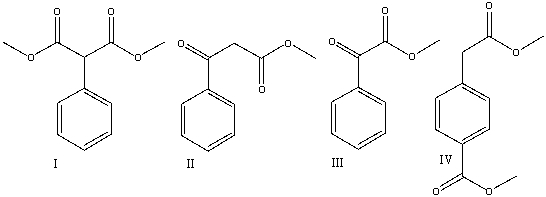
A)I
B)II
C)III
D)IV
E) All of these


A)I
B)II
C)III
D)IV
E) All of these

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
76
Predict the product(s) for the following reaction. 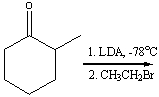
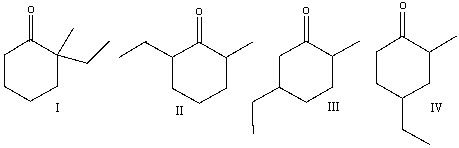
A)I
B)II
C) III
D)IV
E) none of these


A)I
B)II
C) III
D)IV
E) none of these

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
77
Provide the reactant(s) that would give the following possible aldol condensation product. 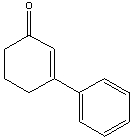


Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
78
Predict the major product for the following reaction. 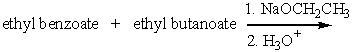
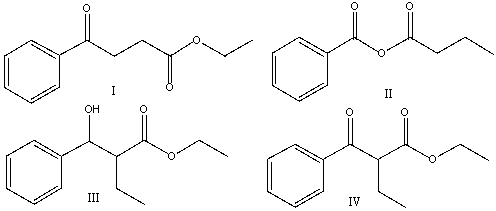
A) I
B) II
C) III
D) IV
E) none of these

A) I
B) II
C) III
D) IV
E) none of these

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
79
Predict the product(s) for the following reaction. 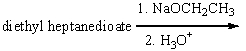
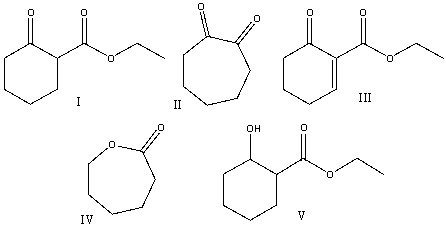
A)I
B)II
C)III
D)IV
E) V
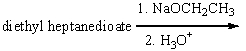

A)I
B)II
C)III
D)IV
E) V

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
80
Predict the product(s) for the following reaction. 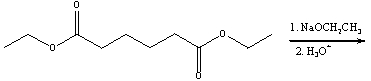
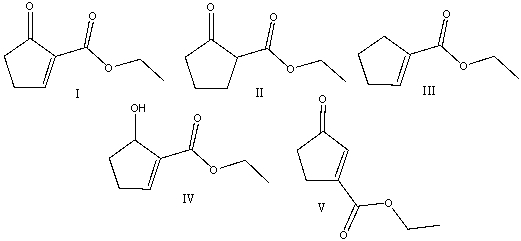
A)I
B)II
C)III
D)IV
E) V


A)I
B)II
C)III
D)IV
E) V

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck



