Deck 21: Carboxylic Acids and Their Derivatives
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/117
Play
Full screen (f)
Deck 21: Carboxylic Acids and Their Derivatives
1
Provide the structure for 5-chloro-3-isopropyl-octanoic acid
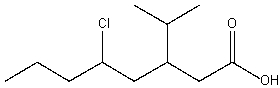
2
Which one of the following compounds is the strongest acid? 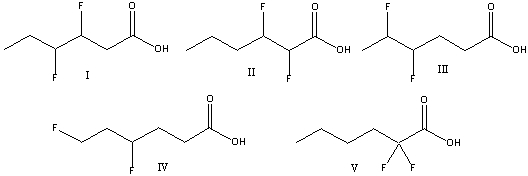
A)I
B)II
C)III
D)IV
E) V

A)I
B)II
C)III
D)IV
E) V
V
3
What is the common name for the following compound? 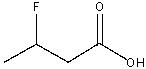
A) 3-fluorobutanoic acid
B) 2-fluorobutanoic acid
C) 3-fluorobutyric acid
D)2-fluorobutyric acid
E) none of these

A) 3-fluorobutanoic acid
B) 2-fluorobutanoic acid
C) 3-fluorobutyric acid
D)2-fluorobutyric acid
E) none of these
3-fluorobutyric acid
4
Which one of the following compounds has the highest boiling point?
A) butanoic acid
B) 1-butanol
C) 2-butanone
D) methoxyethane
E) butanal
A) butanoic acid
B) 1-butanol
C) 2-butanone
D) methoxyethane
E) butanal

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
5
Which of the following is the weakest acid? 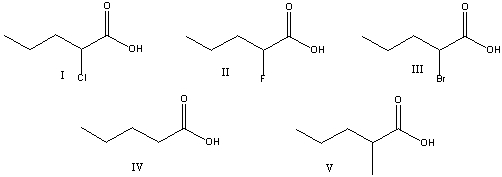
A) I
B)II
C) III
D)IV
E) V

A) I
B)II
C) III
D)IV
E) V

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
6
Methanoic acid is commonly known as ________.

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
7
What is the IUPAC name for the following compound? 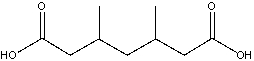
A)2,4-dimethylheptanedioic acid
B)3,5-dimethylheptanedioic acid
C)2,4-dimethylpentanedioic acid
D)3,5-dimethylpentanedioic acid
E)2,4-dimethylheptanoyl acid

A)2,4-dimethylheptanedioic acid
B)3,5-dimethylheptanedioic acid
C)2,4-dimethylpentanedioic acid
D)3,5-dimethylpentanedioic acid
E)2,4-dimethylheptanoyl acid

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
8
What is the IUPAC name for the following compound? 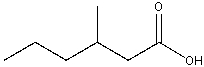
A)2-methylpentanoic acid
B) 3-methylpentanoic acid
C) 2-methylhexanoic acid
D) 3-methylhexanoic acid
E) None of these

A)2-methylpentanoic acid
B) 3-methylpentanoic acid
C) 2-methylhexanoic acid
D) 3-methylhexanoic acid
E) None of these

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
9
What is the IUPAC name for the following compound? 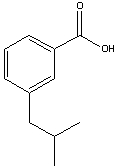


Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
10
Provide the structure for (3S,4S)-3-hydroxy-4-phenylnonanoic acid.

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
11
What is the correct structure for succinic acid 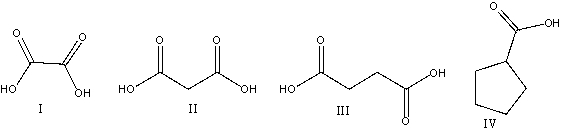
A)I
B)II
C)III
D)IV
E) none of these

A)I
B)II
C)III
D)IV
E) none of these

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
12
What is the IUPAC name for the following compound? 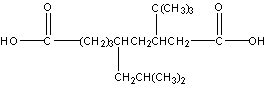


Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
13
Which of the following is the strongest acid? 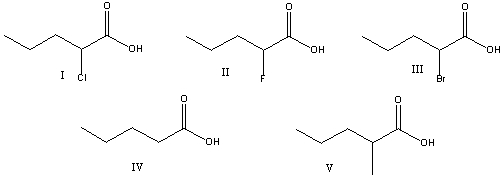
A) I
B)II
C) III
D)IV
E) V

A) I
B)II
C) III
D)IV
E) V

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
14
Ethanoic acid is commonly known as ________.

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
15
What is the IUPAC name for the following compound? 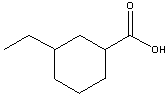


Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
16
Which one of the following compounds has the highest boiling point? 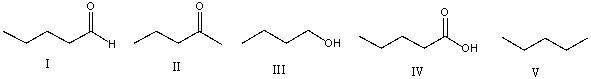
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
17
Which one of the following is the weakest acid?
A) benzoic acid
B) 4-nitrobenzoic acid
C) 4-ethylbenzoic acid
D) 4-chlorobenzoic acid
E) 4-hydroxybenzoic acid
A) benzoic acid
B) 4-nitrobenzoic acid
C) 4-ethylbenzoic acid
D) 4-chlorobenzoic acid
E) 4-hydroxybenzoic acid

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
18
What is the correct structure for trichloroacetic acid? 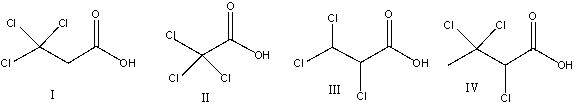
A)I
B)II
C)III
D)IV
E) none of these

A)I
B)II
C)III
D)IV
E) none of these

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
19
Which one of the following is the strongest acid?
A) benzoic acid
B) 4-nitrobenzoic acid
C) 4-ethylbenzoic acid
D) 4-chlorobenzoic acid
E) 4-hydroxybenzoic acid
A) benzoic acid
B) 4-nitrobenzoic acid
C) 4-ethylbenzoic acid
D) 4-chlorobenzoic acid
E) 4-hydroxybenzoic acid

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
20
What is the IUPAC name for the following compound? 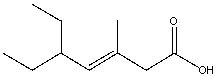


Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
21
What is the IUPAC name for the following compound? 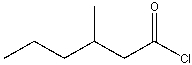
A) -methylvaleryl chloride
B) 2-methylpentanoyl chloride
C) 3-methylhexanoyl chloride
D) 3-methylpentanoyl chloride
E) none of these

A) -methylvaleryl chloride
B) 2-methylpentanoyl chloride
C) 3-methylhexanoyl chloride
D) 3-methylpentanoyl chloride
E) none of these

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
22
Predict the product for the following reaction. 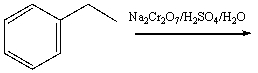
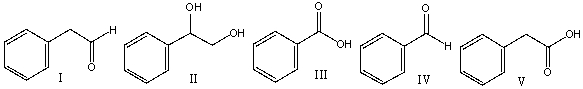
A) I
B) II
C) III
D) IV
E) V
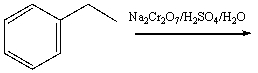

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
23
Predict the product for the following reaction sequence. 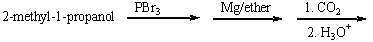
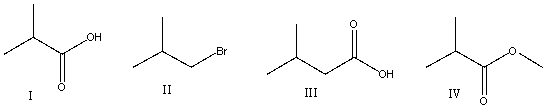
A) I
B) II
C) III
D) IV
E) none of these


A) I
B) II
C) III
D) IV
E) none of these

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
24
Predict the product for the following reaction. 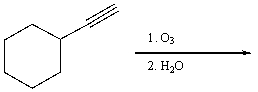
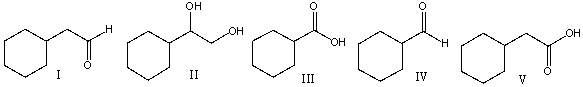
A) I
B) II
C) III
D) IV
E) V
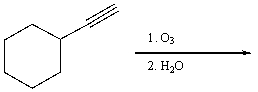

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
25
Provide the reagents necessary to carry out the following conversion. 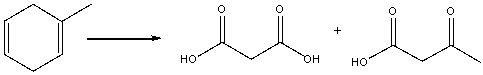


Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
26
Predict the product for the following reaction. 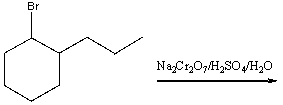
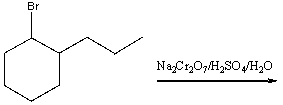

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
27
Predict the product for the following reaction. 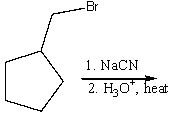


Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
28
Which of the following reagent(s) can be used to carryout the following conversion? 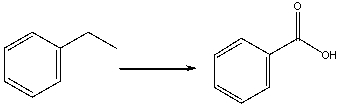
A) PCC/CH2Cl2
B) CO2 followed by H3O+
C) mCPBA
D) Na2Cr2O7/H2SO4/H2O
E) both A & D

A) PCC/CH2Cl2
B) CO2 followed by H3O+
C) mCPBA
D) Na2Cr2O7/H2SO4/H2O
E) both A & D

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
29
Predict the product for the following reaction. 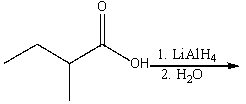
A) 3-methyl-2-pentanone
B) 3-methyl -1-propanol
C) 2-methyl-1-butanol
D) 3-methyl-2-pentanol
E) none of these

A) 3-methyl-2-pentanone
B) 3-methyl -1-propanol
C) 2-methyl-1-butanol
D) 3-methyl-2-pentanol
E) none of these

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
30
Rank the following acids in decreasing (strongest to weakest) order of acidity.
Explain your choice.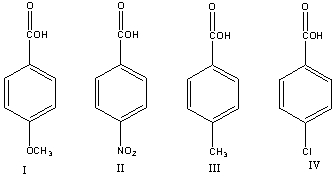
Explain your choice.


Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
31
Provide the reagents necessary to carry out the following conversion. 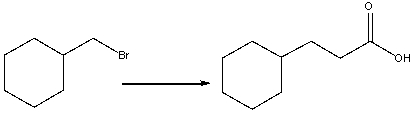


Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
32
Provide the reagents necessary to carry out the following conversion. 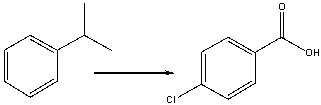


Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
33
Predict the product for the following reaction sequence. 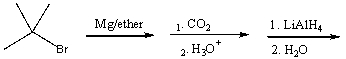
A) 2,2-dimethylpropanoic acid
B) 3,3-dimethyl-2-butanone
C) 2,2-dimethyl-1-propanol
D) 2,2-dimethylbutanoic acid
E) none of these
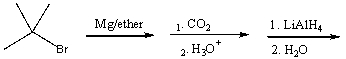
A) 2,2-dimethylpropanoic acid
B) 3,3-dimethyl-2-butanone
C) 2,2-dimethyl-1-propanol
D) 2,2-dimethylbutanoic acid
E) none of these

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
34
Provide the reagents necessary to carry out the following conversion. 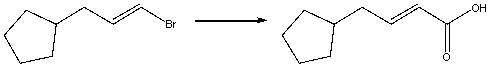


Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
35
Which one of the following is the strongest acid? Explain your choice. 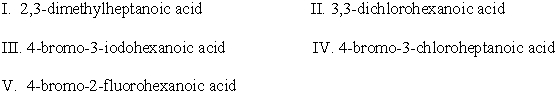 I.
I.
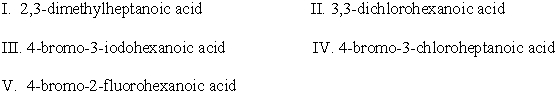 I.
I.
Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
36
Rank the following acids in decreasing (strongest to weakest) order of acidity. 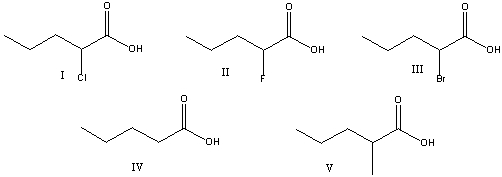
A) V>III>I>II>IV
B)II>I>III>V>IV
C) IV>III>I>II>V
D)IV>V>III>I>II
E) V>I>III>II>IV

A) V>III>I>II>IV
B)II>I>III>V>IV
C) IV>III>I>II>V
D)IV>V>III>I>II
E) V>I>III>II>IV

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
37
Predict the product for the following reaction. 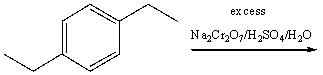


Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
38
Which one of the following is the strongest acid?
A) 2,3-dimethylheptanoic acid
B) 3,3-dichlorohexanoic acid
C) 4-bromo-3-iodohexanoic acid
D) 4-bromo-3-chloroheptanoic acid
E) 4-bromo-2-fluorohexanoic acid
A) 2,3-dimethylheptanoic acid
B) 3,3-dichlorohexanoic acid
C) 4-bromo-3-iodohexanoic acid
D) 4-bromo-3-chloroheptanoic acid
E) 4-bromo-2-fluorohexanoic acid

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
39
Predict the product for the following reaction. 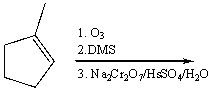
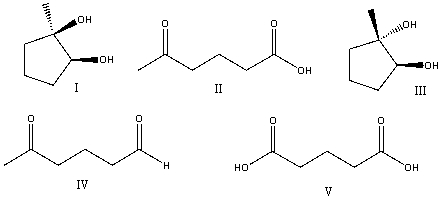
A) I
B) II
C) III
D) IV
E) V
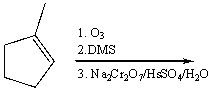

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
40
Provide the reagents necessary to carry out the following conversion. 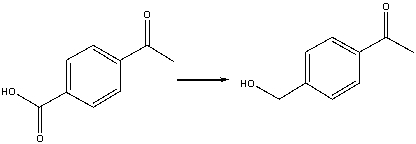


Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
41
Provide the structure for benzoyl chloride.

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
42
What is the IUPAC name for the following compound? 


Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
43
What is the IUPAC name for the following compound? 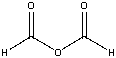
A) methanoic anhydride
B) ethanoic anhydride
C) acetic anhydride
D) both B & C
E) none of these

A) methanoic anhydride
B) ethanoic anhydride
C) acetic anhydride
D) both B & C
E) none of these

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
44
Provide the structure for 4-ethylbenzoyl chloride 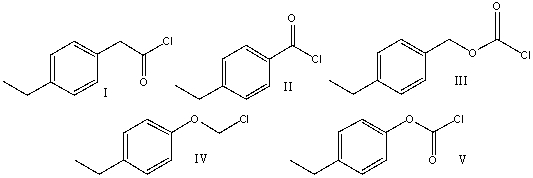
A)I
B) II
C) III
D) IV
E) V

A)I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
45
What is the IUPAC name for the following compound? 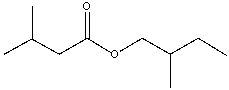
A) 2-methylbutyl 3-methylbutanoate
B) 3-methylbutyl 3-methylbutanoate
C) 2-methylbutyl isovalerate
D) 2-methylbutyl 2-methylbutanoate
E) none of these

A) 2-methylbutyl 3-methylbutanoate
B) 3-methylbutyl 3-methylbutanoate
C) 2-methylbutyl isovalerate
D) 2-methylbutyl 2-methylbutanoate
E) none of these

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
46
What is the IUPAC name for the following compound? 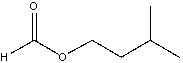
A)2-methylbutyl acetate
B)3-methylbutyl acetate
C)2-methylbutyl methanoate
D)3-methylbutyl methanoate
E) 3-methylbutyl formate

A)2-methylbutyl acetate
B)3-methylbutyl acetate
C)2-methylbutyl methanoate
D)3-methylbutyl methanoate
E) 3-methylbutyl formate

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
47
What is the IUPAC name for the following compound? 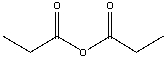


Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
48
Provide the structure for cyclopentanecarbonyl chloride.

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
49
Provide the structure for N-methylcyclohexanecarboxamide.

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
50
Provide the structure for benzyl propionate.

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
51
What is the IUPAC name for the following compound? 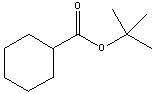


Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
52
What is the IUPAC name for the following compound? 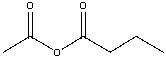
A) methanoic propanoic anhydride
B) methanoic butanoic anhydride
C) ethanoic propionic anhydride
D) ethanoic butanoic anhydride
E) butanoic ethanoic anhydride

A) methanoic propanoic anhydride
B) methanoic butanoic anhydride
C) ethanoic propionic anhydride
D) ethanoic butanoic anhydride
E) butanoic ethanoic anhydride

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
53
What is the IUPAC name for the following compound? 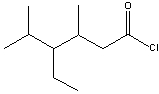
A)3-ethyl-2,4-dimethylpentanoyl chloride
B)4-ethyl-3,5-dimethylpentanoyl chloride
C)3-ethyl-2,4-dimethylhexanoyl chloride
D)4-ethyl-3,5-dimethylhexanoyl chloride
E) none of these

A)3-ethyl-2,4-dimethylpentanoyl chloride
B)4-ethyl-3,5-dimethylpentanoyl chloride
C)3-ethyl-2,4-dimethylhexanoyl chloride
D)4-ethyl-3,5-dimethylhexanoyl chloride
E) none of these

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
54
What is the IUPAC name for the following compound? 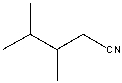
A) 2,3-dimethylbutanenitrile
B) , -dimethylbutyrnitrile
C) , -dimethylbutyrnitrile
D) 3,4-dimethylbutanenitrile
E) 3,4-dimethylpentanenitrile

A) 2,3-dimethylbutanenitrile
B) , -dimethylbutyrnitrile
C) , -dimethylbutyrnitrile
D) 3,4-dimethylbutanenitrile
E) 3,4-dimethylpentanenitrile

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
55
Provide the structure for N-phenyl-N-propyl-2,3-dimethylbutanamide? 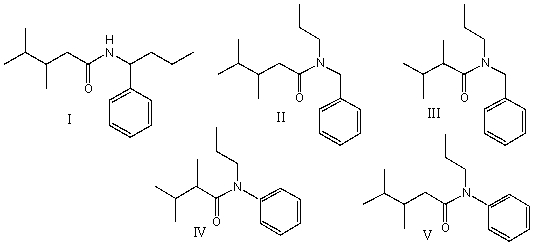
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
56
Provide the structure for acetic formic anhydride.

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
57
What is the common name for the following compound? 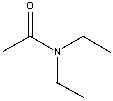


Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
58
What is the IUPAC name for the following compound? 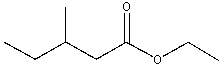
A) sec-butyl ethanoate
B) ethyl 3-methylpentanoate
C) 3-methylbutyl ethanoate
D) ethyl 3-methylbutanoate
E) none of these

A) sec-butyl ethanoate
B) ethyl 3-methylpentanoate
C) 3-methylbutyl ethanoate
D) ethyl 3-methylbutanoate
E) none of these

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
59
Provide the structure for benzoic ethanoic anhydride.

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
60
What is the IUPAC name for the following compound? 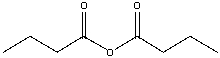
A) propanoic anhydride
B) butanoic anhydride
C) propionic anhydride
D) pentanoic anhydride
E) both A & C

A) propanoic anhydride
B) butanoic anhydride
C) propionic anhydride
D) pentanoic anhydride
E) both A & C

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
61
Predict the product for the following reaction. 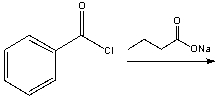
A) I
B) II
C) III
D) IV
E) none of these


A) I
B) II
C) III
D) IV
E) none of these

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
62
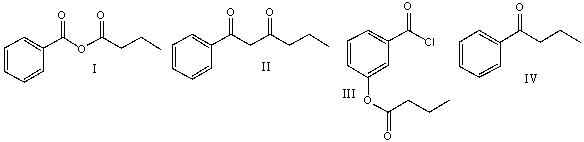
Predict the product for the following reaction. 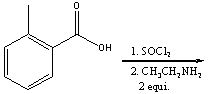


Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
63
Predict the product for the following reaction. 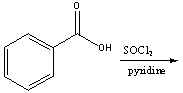
A) I
B) II
C) III
D) IV
E) none of these


A) I
B) II
C) III
D) IV
E) none of these

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
64
Predict the product for the following reaction. 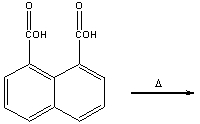


Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
65
Which of the following reactions would not yield isopropyl acetate as major product?
A)

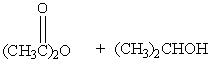
B)

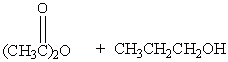
C)
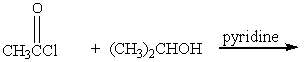
D)
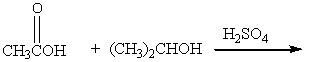
E) A & C
A)

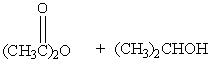
B)

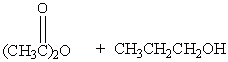
C)
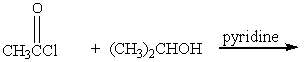
D)
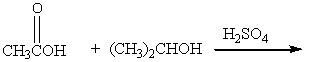
E) A & C

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
66
Provide the reagents necessary to carry out the following conversion. 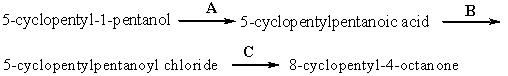
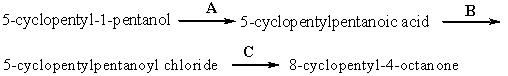

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
67
Predict the product for the following reaction. 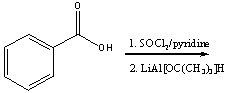
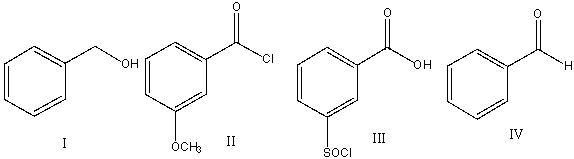
A) I
B) II
C) III
D) IV
E) none of these


A) I
B) II
C) III
D) IV
E) none of these

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
68
Provide the reagents necessary to convert 4,5-dimethyl-1-hexanol into N-cyclopentyl-4,5-dimethylhexanamide

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
69
Predict the product for the following reaction. 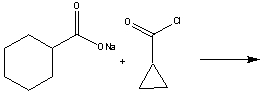


Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
70
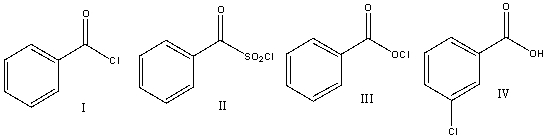
Rank the following carboxylic acid derivatives in decreasing order (most to least) of reactivity towards nucleophilic substitution. 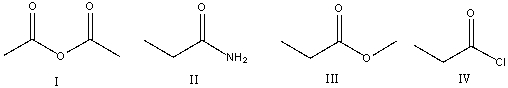
A) I>IV>III>II
B) II>III>IV>I
C) I>III>II>IV
D) III>IV>II>I
E) IV>I>III>II

A) I>IV>III>II
B) II>III>IV>I
C) I>III>II>IV
D) III>IV>II>I
E) IV>I>III>II

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
71
What is the IUPAC name for the following compound? 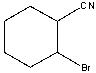


Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
72
Provide the reagents necessary to carry out the following conversion. 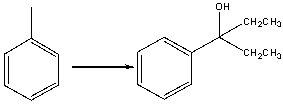


Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
73
Provide a stepwise synthesis for the following compound using benzyl alcohol as your only source of carbon and using any other reagents necessary. 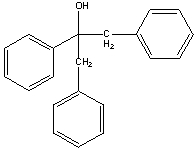


Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
74
Predict the product for the following reaction. 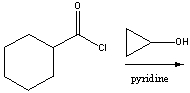


Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
75
Predict the product for the following reaction. 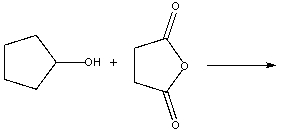


Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
76
Predict the product for the following reaction. 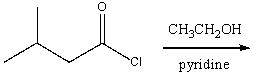
A) ethyl 3-methylbutanoate
B) ethyl 2-methylpropanoate
C) isobutyl ethanoate
D) 5-methyl-3-hexanone
E) none of these

A) ethyl 3-methylbutanoate
B) ethyl 2-methylpropanoate
C) isobutyl ethanoate
D) 5-methyl-3-hexanone
E) none of these

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
77
Predict the product for the following reaction. 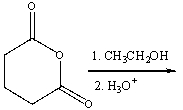
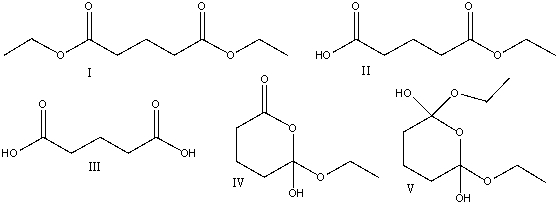
A) I
B) II
C) III
D) IV
E) V


A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
78
Predict the product for the following reaction. 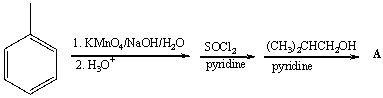
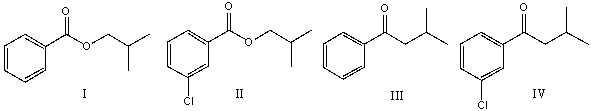
A) I
B) II
C) III
D) IV
E) none of these


A) I
B) II
C) III
D) IV
E) none of these

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
79
Provide the structure for 3-ethylbenzonitrile.

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
80
Rank the following carboxylic acid derivatives in decreasing order (most to least) of reactivity towards nucleophilic substitution. 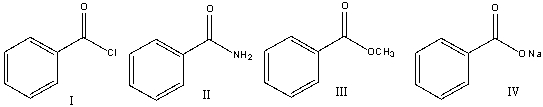
A) I>IV>III>II
B) II>III>IV>I
C) I>III>II>IV
D) III>IV>II>I
E) IV>I>III>II

A) I>IV>III>II
B) II>III>IV>I
C) I>III>II>IV
D) III>IV>II>I
E) IV>I>III>II

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck



