Deck 11: Radical Reactions
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/90
Play
Full screen (f)
Deck 11: Radical Reactions
1
Use correct arrow formalism to show the mechanism of the following radical process: 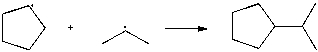
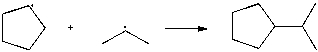
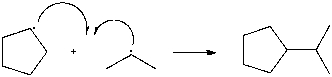
2
Use correct arrow formalism to show the mechanism of the following radical process: 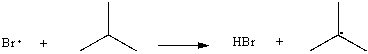

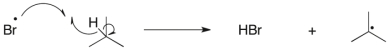
3
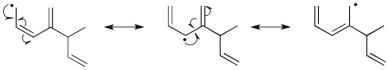
Use correct arrow formalism to draw all of the reasonable resonance structures for the radical shown below. 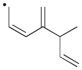

4
Which of the following is the most stable radical?
A)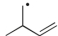
B)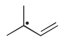
C)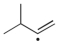
D)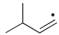
E)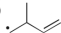
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
5
Which term most accurately describes the process shown below? 
A) coupling
B) proton transfer
C) halogen abstraction
D) hydrogen abstraction
E) homolytic cleavage

A) coupling
B) proton transfer
C) halogen abstraction
D) hydrogen abstraction
E) homolytic cleavage

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
6
Rank the following radicals in order of decreasing stability (most stable to least stable). 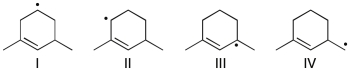
A) IV > I > II > III
B) III > I > II > IV
C) III > II > I > IV
D) III > IV > II > I
E) II > III > I > IV

A) IV > I > II > III
B) III > I > II > IV
C) III > II > I > IV
D) III > IV > II > I
E) II > III > I > IV

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
7
Which of the following is an example of termination?
A)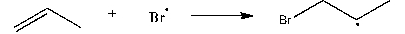
B)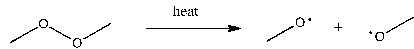
C)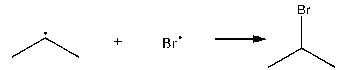
D)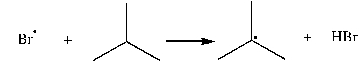
E)
A)
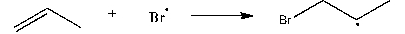
B)
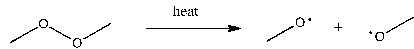
C)
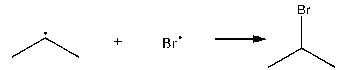
D)

E)

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
8
Using correct arrow formalism, draw all of the reasonable resonance structures for the radical shown below. 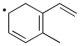


Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
9
Which term most accurately describes the process shown below? 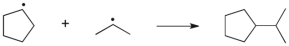
A) hydrogen abstraction
B) halogen abstraction
C) homolytic cleavage
D) coupling
E) elimination
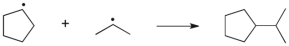
A) hydrogen abstraction
B) halogen abstraction
C) homolytic cleavage
D) coupling
E) elimination

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
10
Use correct arrow formalism to show the mechanism of the following radical process: 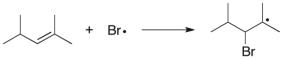
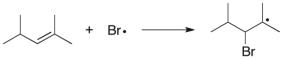

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
11
In the molecule shown below, determine which of the highlighted C-H bonds (from a to e) is expected to have the lowest bond dissociation energy. 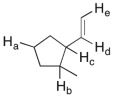
A) C-Ha
B) C-Hb
C) C-Hc
D) C-Hd
E) C-He

A) C-Ha
B) C-Hb
C) C-Hc
D) C-Hd
E) C-He

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
12
A bromine radical can add to the bond of 2-methylpropene. Use correct arrow formalism to show this process and the expected result.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
13
Which of the following are the products of a homolytic cleavage of a C-C bond of ethane?
A)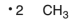
B)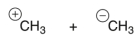
C)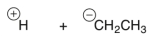
D)
A)
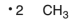
B)
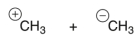
C)
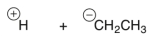
D)


Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
14
Which term most accurately describes the process shown below? 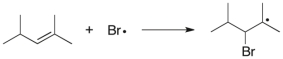
A) coupling
B) hydrogen abstraction
C) halogen abstraction
D) homolytic cleavage
E) addition to a bond
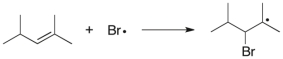
A) coupling
B) hydrogen abstraction
C) halogen abstraction
D) homolytic cleavage
E) addition to a bond

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
15
Which of the labeled C-H bonds is the weakest? 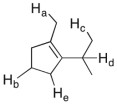
A) C-Ha
B) C-Hb
C) C-Hc
D) C-Hd
E) C-He

A) C-Ha
B) C-Hb
C) C-Hc
D) C-Hd
E) C-He

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
16
Use correct arrow formalism consistent with the following radical process: 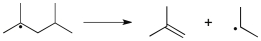


Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
17
Use correct arrow formalism to show a homolytic bond cleavage of ethane to produce two methyl radicals.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
18
Which term most accurately describes the process shown below? 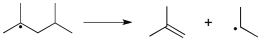
A) coupling
B) elimination
C) halogen abstraction
D) hydrogen abstraction
E) homolytic cleavage

A) coupling
B) elimination
C) halogen abstraction
D) hydrogen abstraction
E) homolytic cleavage

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
19
Rank the following radicals in order of decreasing stability (most stable to least stable). 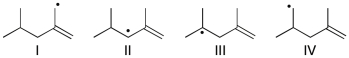
A) IV > I > II > III
B) III > I > II > IV
C) III > II > I > IV
D) III > IV > II > I
E) II > I > III > IV

A) IV > I > II > III
B) III > I > II > IV
C) III > II > I > IV
D) III > IV > II > I
E) II > I > III > IV

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
20
Which of the following is an example of initiation?
A)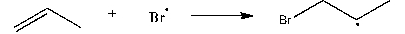
B)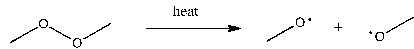
C)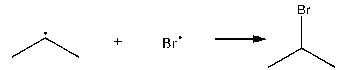
D)
E)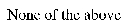
A)
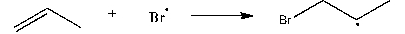
B)
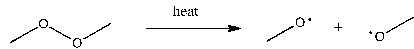
C)
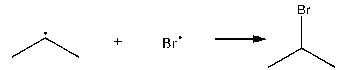
D)

E)

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
21
Use correct arrow formalism to show the propagation steps in the chlorination of propane to produce 2-chloropropane.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
22
Which of the following would you expect to function as an initiator at the lowest temperature?
A)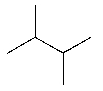
B) Cl2
C)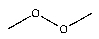
D)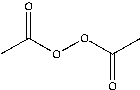
A)

B) Cl2
C)
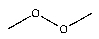
D)


Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
23
Predict the major product(s) of the following reaction. 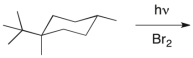


Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
24
Which of the following is the correct name for the major product of the following reaction? 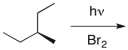
A) (R)-3-bromo-3-ethylbutane
B) (S)-3-bromo-3-ethylbutane
C) (R)-3-bromo-3-methylpentane
D) (S)-3-bromo-3-methylpentane
E) 3-bromo-3-methylpentane

A) (R)-3-bromo-3-ethylbutane
B) (S)-3-bromo-3-ethylbutane
C) (R)-3-bromo-3-methylpentane
D) (S)-3-bromo-3-methylpentane
E) 3-bromo-3-methylpentane

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
25
Predict the major product(s) of the following reaction. 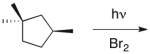


Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
26
Which of the following correctly describes the nature of the transition state of the rate-determining step of the free-radical bromination of methane?
A) the transition state resembles the reactants more than the products
B) the transition state resembles the products more than the reactants
C) the transition state equally resembles products and reactants
A) the transition state resembles the reactants more than the products
B) the transition state resembles the products more than the reactants
C) the transition state equally resembles products and reactants

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
27
Cyclic compound A has molecular formula C5H10 and undergoes monochlorination to yield exactly three different constitutional isomers. Identify compound A and show the monochlorination products.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
28
Free radical chlorination of ethane can produce higher halogenation products (dichlorinated, trichlorinated, etc…) in addition to chloroethane. How could the production of higher halogenated products be minimized?
A) Use an excess of chlorine
B) Use an excess of ethane
C) Use equimolar chlorine and ethane
D) It is not possible to minimize the production of higher halogenated products
A) Use an excess of chlorine
B) Use an excess of ethane
C) Use equimolar chlorine and ethane
D) It is not possible to minimize the production of higher halogenated products

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
29
Both compounds A and B have molecular formula C6H14. Monochlorination of compound A results in formation of two constitutional isomers. Monochlorination of compound B results in formation of four constitutional isomers. Identify compounds A and B, and show the products of each monochlorination.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
30
Which of the following is most reactive towards chlorination?
A) methane
B) chloromethane
C) dichloromethane
D) chloroform
E) ethane
A) methane
B) chloromethane
C) dichloromethane
D) chloroform
E) ethane

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
31
Predict the major product(s) of the following reaction:
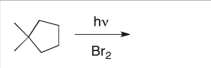
A)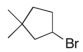
B)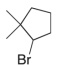
C)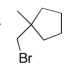
D)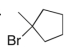

A)

B)

C)

D)


Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
32
Which of the following is the rate-determining step in the free-radical bromination of methane?
A)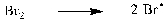
B)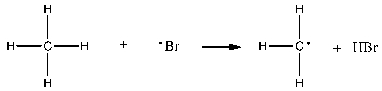
C)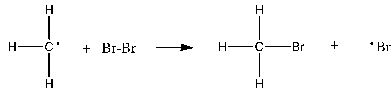
D)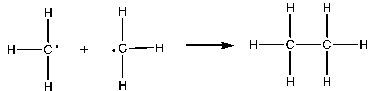
A)
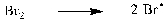
B)

C)

D)


Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
33
Which of the following are possible termination steps in the chlorination of methane?
A)
B)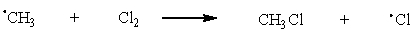
C)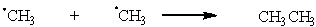
D)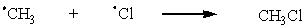
A)

B)
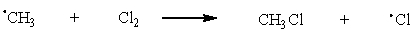
C)
D)

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
34
Which of the following shows the initiation step of monochlorination of methane? 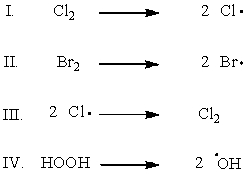
A) I
B) II
C) III
D) IV
E) I and II

A) I
B) II
C) III
D) IV
E) I and II

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
35
Predict the major product(s) of the following reaction. 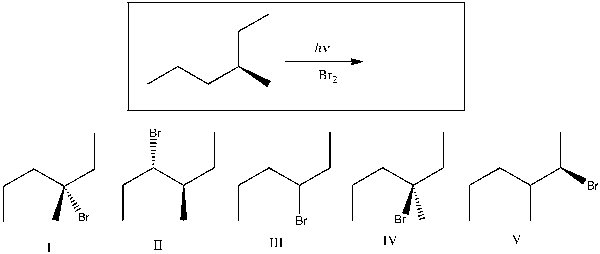
A) I
B) II and IV
C) III and V
D) I and IV
E) V

A) I
B) II and IV
C) III and V
D) I and IV
E) V

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
36
Predict the major product obtained upon radical bromination of t-butylcyclohexane.
A) 1-bromo-1-tert-butylcyclohexane
B) 2-bromo-1-tert-butylcyclohexane
C) 3-bromo-1-tert-butylcyclohexane
D) 4-bromo-1-tert-butylcyclohexane
E) (1-bromo-1,1-dimethyl)ethylcyclohexane
A) 1-bromo-1-tert-butylcyclohexane
B) 2-bromo-1-tert-butylcyclohexane
C) 3-bromo-1-tert-butylcyclohexane
D) 4-bromo-1-tert-butylcyclohexane
E) (1-bromo-1,1-dimethyl)ethylcyclohexane

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
37
Which of the following processes is responsible for the fact that free radical bromination of methane is slower than free radical chlorination?
A) initiation
B) hydrogen abstraction
C) halogen abstraction
D) termination
E) entropy
A) initiation
B) hydrogen abstraction
C) halogen abstraction
D) termination
E) entropy

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
38
Predict the major product of the following reaction:
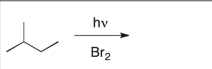
A)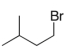
B)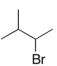
C)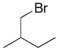
D)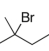

A)

B)

C)

D)


Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
39
How many constitutional isomers are possible if propane is dichlorinated? Draw them.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
40
Which of the following represents a propagation step in the monochlorination of methylene chloride (CH2Cl2)?
A)
B)Cl2
C)
D)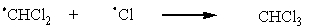
A)

B)Cl2
C)

D)

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
41
Upon treatment with NBS and irradiation with UV light, 2-methyl-2-butene reacts to produce exactly six monobrominated compounds. Draw the products of this reaction.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
42
Upon treatment of 1-methylcyclopentene with NBS and irradiation with UV light, exactly nine compounds (including stereoisomers) are formed. Draw all nine products.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
43
Predict the major product(s) of the following reaction. 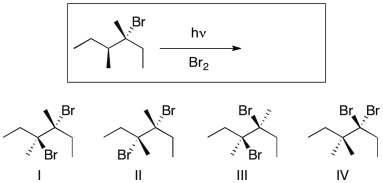
A) I
B) I and II
C) I, II, and III
D) I, II, III
E) IV

A) I
B) I and II
C) I, II, and III
D) I, II, III
E) IV

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
44
Which of the following shows the correct products initially formed (first step) when ozone absorbs ultraviolet light?
A)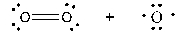
B)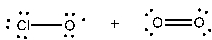
C)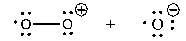
D)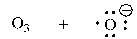
A)
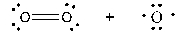
B)
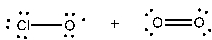
C)
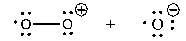
D)
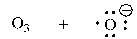

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
45
Use correct arrow formalism to show the second propagation step for the reaction of a chlorine radical with ozone.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
46
Draw the major product(s) of the following reaction. Is the product optically active? Explain. 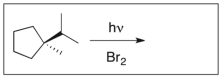


Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
47
Which term best describes the process shown below? 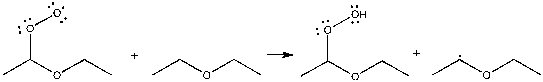
A) neutralization
B) propagation
C) termination
D) initiation
E) elimination

A) neutralization
B) propagation
C) termination
D) initiation
E) elimination

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
48
Which of the following compounds would be expected to be least destructive to the ozone layer?
A)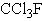
B)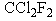
C)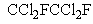
D)
A)
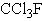
B)
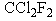
C)
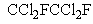
D)


Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
49
Compound A has molecular formula C9H20. Compound A produces exactly three constitutional isomers upon monochlorination, and one major constitutional isomer upon monobromination. Which of the following are possible structures of compound A? 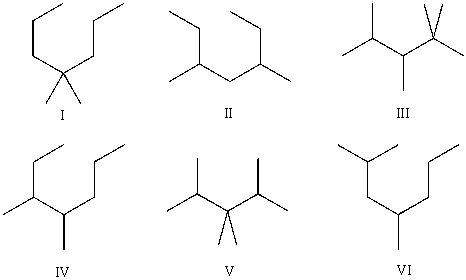


Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
50
Which of the following is expected to be a major product for the reaction shown below? 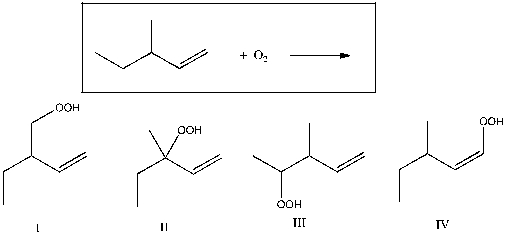
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
51
What reagents would best accomplish the following transformation? 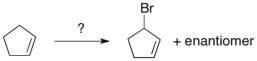
A) Br2
B) PBr3
C) CH3Br
D) NBS, heat

A) Br2
B) PBr3
C) CH3Br
D) NBS, heat

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
52
Use correct arrow formalism to show the propagation steps for the autooxidation of diethyl ether.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
53
Upon treatment with NBS and irradiation with UV light, 1-ethyl-4-methylbenzene produces exactly three monobrominated compounds (including stereoisomers). Draw the products of this reaction.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
54
Upon treatment with NBS and irradiation with UV light, 2-propyl-1-pentene produces exactly four monobrominated compounds (including stereoisomers). Draw and name the products of this reaction.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
55
Which of the following are possible product(s) of the reaction shown? 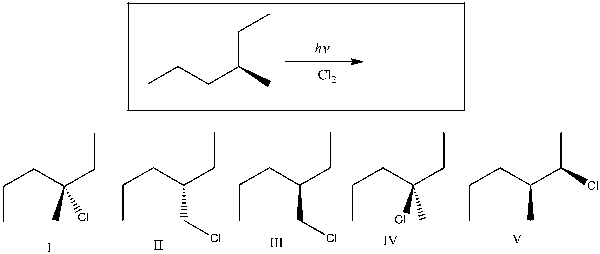
A) I
B) I, II
C) I, II, IV, V
D) I, III, IV, V
E) I, II, III, IV, IV

A) I
B) I, II
C) I, II, IV, V
D) I, III, IV, V
E) I, II, III, IV, IV

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
56
Which of the following are major products of the reaction shown? 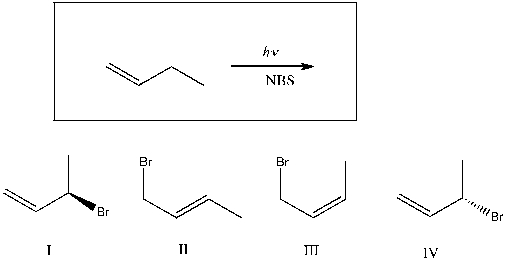
A) I
B) I, II
C) I, IV
D) I, III
E) I, II, III, IV

A) I
B) I, II
C) I, IV
D) I, III
E) I, II, III, IV

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
57
Draw the major product(s) of the following reaction. Is the product optically active? Explain. 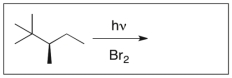


Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
58
Which of the following is (are) the likely major product(s) of the reaction shown? 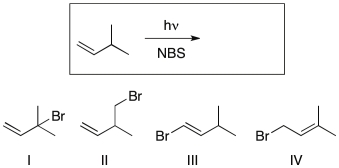
A) I
B) II
C) III
D) I and III
E) I and IV

A) I
B) II
C) III
D) I and III
E) I and IV

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
59
Which of the following is expected to function as an antioxidant? 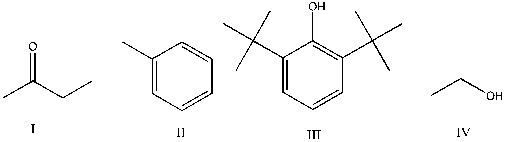
A) I
B) II
C) III
D) IV
E) I, II, III, and IV

A) I
B) II
C) III
D) IV
E) I, II, III, and IV

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
60
Upon treatment with NBS and irradiation with UV light, 2-isopropyl-3-methyl-1-butene reacts to produce exactly two monobrominated compounds. Draw the products of this reaction.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
61
Thermal cracking of butane can produce ethyl radicals via homolytic cleavage. Use correct arrow formalism to show this process.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
62
Predict the major product(s) of the reaction shown below: 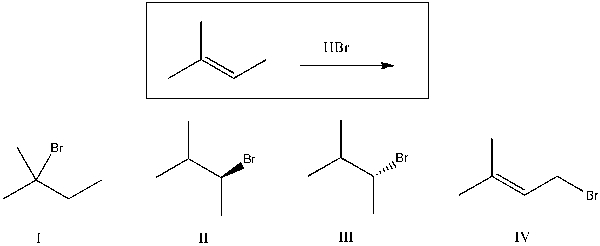
A) I
B) II
C) III
D) IV
E) II and III

A) I
B) II
C) III
D) IV
E) II and III

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
63
Which term best describes the process shown below? 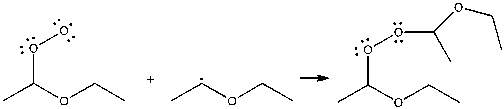
A) initiation
B) propagation
C) termination
D) elimination
E) inhibition

A) initiation
B) propagation
C) termination
D) elimination
E) inhibition

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
64
Your textbook mentions that the free radical polymerization of ethylene can produce branches. Although branches of various lengths are possible, butyl branches are very common. Suggest an explanation for this fact.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
65
One possible product of thermal cracking of hexane is 1-butene. Use correct arrow formalism to suggest a possible mechanism for this process starting from an alkyl radical.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
66
Predict the major product(s) of the reaction shown below: 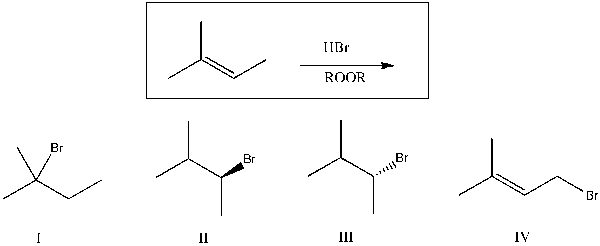
A) I
B) II
C) III
D) IV
E) II and III

A) I
B) II
C) III
D) IV
E) II and III

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
67
Predict the product(s) of the following reaction: 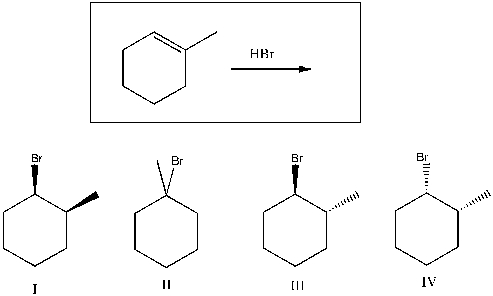
A) I
B) II
C) III
D) I, II
E) I, II, III, IV

A) I
B) II
C) III
D) I, II
E) I, II, III, IV

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
68
The free radical polymerization of styrene with benzoyl peroxide yields a polymer that has repeat units arranged primarily in a 'head-to-tail' arrangement. This means that the phenyl group primarily ends up placed at alternating carbon atoms along the chain. Use correct arrow formalism to show why this arrangement is preferred over a 'head-to-head' or 'tail-to-tail' arrangement. 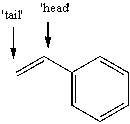


Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
69
Azobisisobutyronitrile (AIBN) is commonly used as a radical initiator. Use correct arrow formalism to show this process. 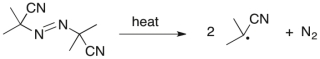


Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
70
Which monomer is used for the synthesis of Teflon?
A) 1,1-difluoroethene
B) 1,1,2,2-tetrafluoropropene
C) 1,1,2,2-tetrafluoroethene
D) tetrafluoromethane
A) 1,1-difluoroethene
B) 1,1,2,2-tetrafluoropropene
C) 1,1,2,2-tetrafluoroethene
D) tetrafluoromethane

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
71
Predict the product(s) of the following reaction: 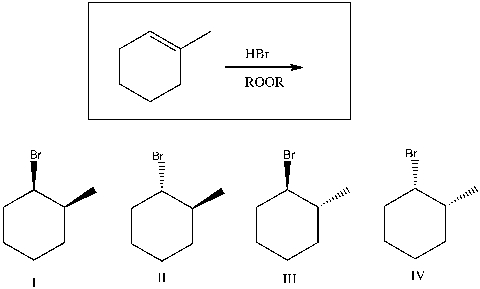
A) I
B) II
C) III
D) I, II
E) I, II, III, IV

A) I
B) II
C) III
D) I, II
E) I, II, III, IV

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
72
Which intermediate leads to the major product for the reaction of 2-methyl-2-butene with hydrogen bromide? 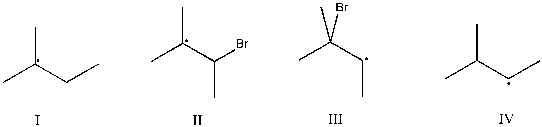
A) I
B) II
C) III
D) IV
E) None of the above

A) I
B) II
C) III
D) IV
E) None of the above

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
73
Use correct arrow formalism to show termination by coupling of two growing poly(vinyl chloride) chains.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
74
Draw the product of coupling of the following radicals. 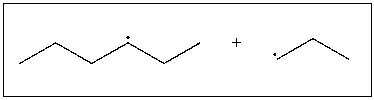


Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
75
Of the four choices shown, which is likely to be a major product of the reaction below? 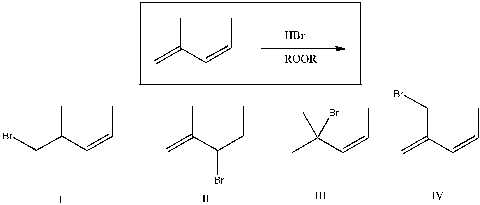
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
76
Which intermediate leads to the major product for the reaction of 2-methyl-2-butene with hydrogen bromide and hydrogen peroxide? 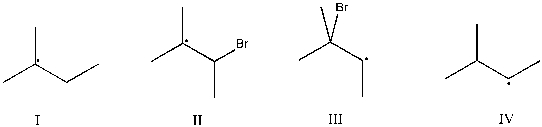
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
77
Which monomer is used for the synthesis of poly(vinyl chloride)?
A) 1-chloroethene
B) 1-chloroethane
C) 1,2-dichloroethene
D) 1-chloro-1-propene
A) 1-chloroethene
B) 1-chloroethane
C) 1,2-dichloroethene
D) 1-chloro-1-propene

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
78
Which of the following steps is thermodynamically unfavorable at all temperatures?
A)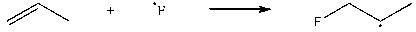
B)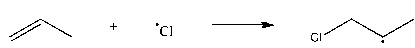
C)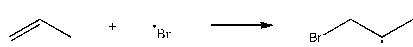
D)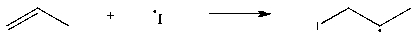
A)
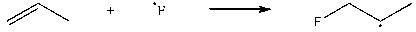
B)
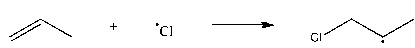
C)
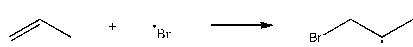
D)
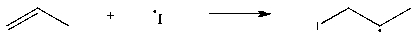

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
79
Compound A, C6H12 reacts with HBr/ROOR to give compound B, C6H13Br. Compound C, C6H14, reacts with bromine and light to produce compound B, C6H13Br. Suggest structures for compounds A, B, and C.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
80
Propylene (propene) undergoes free radical polymerization with benzoyl peroxide, but does not produce very long chains. Provide a reasonable explanation for this result.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck



