Deck 9: Alkynes
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/124
Play
Full screen (f)
Deck 9: Alkynes
1
What is the correct IUPAC name for the following structure? 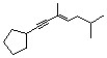
A) (Z) 1-cyclopentyl-3,6-dimethylhept-3-en-1-yne
B) (E) 1-cyclopentyl-3,6-dimethylhept-1-yn-3-ene
C) (Z) 1-cyclopentyl-4,7-dimethylhept-4-en-2-yne
D) (E) 1-cyclopentyl-3,6-dimethylhept-3-en-1-yne

A) (Z) 1-cyclopentyl-3,6-dimethylhept-3-en-1-yne
B) (E) 1-cyclopentyl-3,6-dimethylhept-1-yn-3-ene
C) (Z) 1-cyclopentyl-4,7-dimethylhept-4-en-2-yne
D) (E) 1-cyclopentyl-3,6-dimethylhept-3-en-1-yne
(E) 1-cyclopentyl-3,6-dimethylhept-3-en-1-yne
2
How many distinct internal alkynes exist with a molecular formula of C6H10?
A) 1
B) 2
C) 3
D) 4
E) 5
A) 1
B) 2
C) 3
D) 4
E) 5
3
3
Provide the IUPAC name for the compound below. 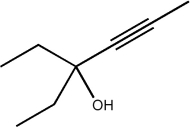

3-ethylhex-4-yn-3-ol
4
Draw an acceptable structure for 3-sec-butylhept-1-yne.

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
5
How many distinct terminal alkynes exist with a molecular formula of C5H8?
A) 1
B) 2
C) 3
D) 4
E) 5
A) 1
B) 2
C) 3
D) 4
E) 5

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
6
Name the compound shown below. 


Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
7
Give the IUPAC name for BrCH2CH2C=CCH2CH3.

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
8
Draw an acceptable structure for hex- 2-yne.

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
9
Provide the IUPAC name for Cl3C(CH2)3C≡CH.

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
10
Give the IUPAC name for (CH3)2C(CH2CH3)C=CCH(CH3)2.

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
11
Provide the IUPAC name for (CH3)2CHCH2CH(OH)CH2C≡CCH3.

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
12
Give the IUPAC name for CH3CH2C=CCH(OH)CH3.

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
13
How many distinct alkynes exist with a molecular formula of C4H8?
A) 0
B) 1
C) 2
D) 3
E) 4
A) 0
B) 1
C) 2
D) 3
E) 4

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
14
Draw an acceptable structure for (S)-5-phenyloct-2-yne.

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
15
Draw an acceptable structure for hepta-3,6-dien-1-yne.

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
16
Draw an acceptable structure for acetylene.

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
17
Draw and name all terminal alkynes with molecular formula C5H8.

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
18
Give the IUPAC name for HC=CCH2CH2CH3.

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
19
Give the IUPAC name for Cl3CCH2CH2CH2C≡CH.

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
20
How many distinct alkynes exist with a molecular formula of C4H6?
A) 0
B) 1
C) 2
D) 3
E) 4
A) 0
B) 1
C) 2
D) 3
E) 4

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
21
The carbon-carbon triple bond of an alkyne is composed of ________.
A) three σ bonds
B) three π bonds
C) two σ bonds and one π bond
D) one σ bond and two π bonds
A) three σ bonds
B) three π bonds
C) two σ bonds and one π bond
D) one σ bond and two π bonds

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
22
In trans-hept-4-en-2-yne the shortest carbon-carbon bond is between carbons ________.
A) 1 and 2
B) 2 and 3
C) 3 and 4
D) 4 and 5
E) 6 and 7
A) 1 and 2
B) 2 and 3
C) 3 and 4
D) 4 and 5
E) 6 and 7

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
23
Which of the species below is less basic than acetylide?
A) CH3Li
B) CH3ONa
C) CH3MgBr
D) both A and C
E) all of the above
A) CH3Li
B) CH3ONa
C) CH3MgBr
D) both A and C
E) all of the above

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
24
What is the pKa of a terminal alkyne?
A) 4
B) 10
C) 16
D) 25
E) 44
A) 4
B) 10
C) 16
D) 25
E) 44

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
25
Provide the major organic product of the reaction below. 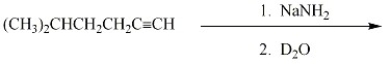
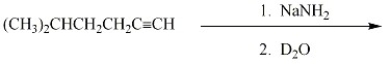

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
26
Why are terminal alkynes more acidic then other hydrocarbons?

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
27
Which of the following describes the physical properties of an alkyne?
A) relatively nonpolar
B) nearly insoluble in water
C) less dense than water
D) insoluble in most organic solvents
E) boiling point nearly the same as an alkane with similar carbon skeleton
A) relatively nonpolar
B) nearly insoluble in water
C) less dense than water
D) insoluble in most organic solvents
E) boiling point nearly the same as an alkane with similar carbon skeleton

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
28
How many moles of oxygen are required in the complete combustion of 1 mole of acetylene?
A) 1
B) 1.5
C) 2
D) 2.5
E) 3
A) 1
B) 1.5
C) 2
D) 2.5
E) 3

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
29
The pi bond of an alkyne is ________ and ________ than the pi bond of an alkene.
A) shorter, stronger
B) shorter, weaker
C) longer, stronger
D) longer, weaker
A) shorter, stronger
B) shorter, weaker
C) longer, stronger
D) longer, weaker

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
30
What is the correct IUPAC name for the following compound? 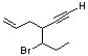
A) 4-(1-bromopropyl)-1-hexen-5-yne
B) 4-allyl-5-bromo-1-hexyne
C) 3-(1-bromopropyl)-5-hexen-1-yne
D) 5-bromo-4-ethynyl-1-heptene

A) 4-(1-bromopropyl)-1-hexen-5-yne
B) 4-allyl-5-bromo-1-hexyne
C) 3-(1-bromopropyl)-5-hexen-1-yne
D) 5-bromo-4-ethynyl-1-heptene

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
31
Among the compounds water, but-1-yne, but-2-yne, and ethane, which are stronger acids than ammonia?
A) but-1-yne and ethane
B) water and but-1-yne
C) water and ethane
D) but-1-yne and but-2-yne
A) but-1-yne and ethane
B) water and but-1-yne
C) water and ethane
D) but-1-yne and but-2-yne

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
32
How many moles of water are produced when one mole of propyne undergoes complete combustion?

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
33
Explain why the synthetic route shown below would be unsuccessful.
H-C≡C:- Na+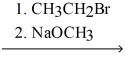 H3CH2C-C≡C-CH2CH3
H3CH2C-C≡C-CH2CH3
3. CH3CH2I
H-C≡C:- Na+
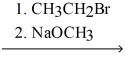 H3CH2C-C≡C-CH2CH3
H3CH2C-C≡C-CH2CH33. CH3CH2I

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
34
To a solution of propyne in diethyl ether, one molar equivalent of CH3Li was added and the resulting mixture was stirred for 0.5 hour. After this time, an excess of D2O was added. Describe the major organic product(s) of this reaction.
A) CH3C≡CD + CH4
B) CH3C≡CCH3
C) CD3C≡CD3
D) CH3C≡CCD3
E) CH3C≡CD + CH3D
A) CH3C≡CD + CH4
B) CH3C≡CCH3
C) CD3C≡CD3
D) CH3C≡CCD3
E) CH3C≡CD + CH3D

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
35
Circle the shortest bond in the compound below. 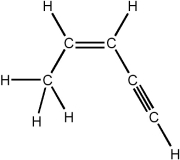


Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
36
Provide the IUPAC name for the compound below. 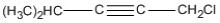
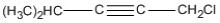

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
37
The correct IUPAC name for the following structure is ________. 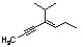
A) (Z) 4-isopropyl-4-hepten-2-yne
B) (E) 4-isopropyl-3-hepten-5-yne
C) (E) 4-isopropyl-4-hepten-2-yne
D) (Z) 4-isopropyl-3-hepten-5-yne

A) (Z) 4-isopropyl-4-hepten-2-yne
B) (E) 4-isopropyl-3-hepten-5-yne
C) (E) 4-isopropyl-4-hepten-2-yne
D) (Z) 4-isopropyl-3-hepten-5-yne

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
38
Give the IUPAC name for CH3CH=CHCH=CHC≡CCH3

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
39
Which of the following alkynes has the lowest boiling point?
A) 3,3-dimethyl-1-butyne
B) 1-hexyne
C) 2-hexyne
D) 3-hexyne
E) 1-decyne
A) 3,3-dimethyl-1-butyne
B) 1-hexyne
C) 2-hexyne
D) 3-hexyne
E) 1-decyne

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
40
What products result when calcium carbide is combined with water?

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
41
Provide the structure of the major organic product(s) in the reaction sequence below.
CH3CH2C≡CH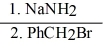 →
→
CH3CH2C≡CH
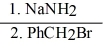 →
→
Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
42
Describe a sequence of reactions by which butylbenzene can be straightforwardly prepared from phenylacetylene.

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
43
Provide the structure of the major organic product(s) in the reaction below. 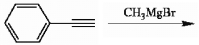
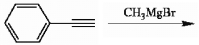

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
44
Explain why the synthetic route shown below would be unsuccessful.
H≡C≡C:-Na+
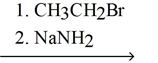
C-C≡C-CH2CH3
3. (CH3)3CBr
H≡C≡C:-Na+
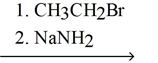
C-C≡C-CH2CH3
3. (CH3)3CBr

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
45
Provide a correct structure for the product of the following reaction. 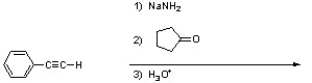


Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
46
Describe a sequence of reactions by which hept-3-yne can be straightforwardly prepared from acetylene.

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
47
Name the product which results when CH3C=CLi reacts with CH3CH2COCH2CH3 followed by addition of water.

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
48
Describe a sequence of reactions by which 1-propylcyclohexan-1-ol can be straightforwardly prepared from propyne.

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
49
Provide the structure of the major organic product(s) in the reaction below. 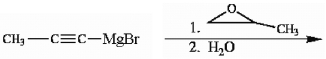
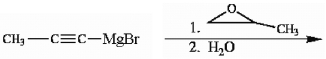

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
50
What is the pKa of a terminal alkyne?
A) 4
B) 10
C) 16
D) 25
E) 44
A) 4
B) 10
C) 16
D) 25
E) 44

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
51
Describe a sequence of reactions by which the compound shown below can be straightforwardly prepared from acetylene. 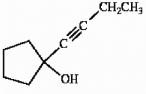


Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
52
Describe a qualitative, nonspectroscopic means for distinguishing terminal alkynes from internal ones.

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
53
What's the problem with the synthetic approach shown below? 


Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
54
What conditions could be used to isomerize hept-2-yne to hept-1-yne?

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
55
Provide the structure of the major organic product(s) in the reaction sequence below. 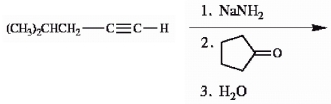
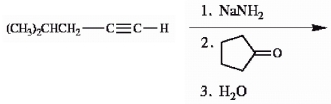

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
56
Which of the following bases will favorably deprotonate a terminal alkyne?
(More than one answer is possible.)
A) n-BuLi
B) LDA
C) NaOMe
D) PhMgBr (Ph=phenyl)
(More than one answer is possible.)
A) n-BuLi
B) LDA
C) NaOMe
D) PhMgBr (Ph=phenyl)

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
57
Complete the short synthesis below by providing the necessary reagents. 


Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
58
2-Methylhex-3-yne can be prepared by the reaction of an alkynide with an alkyl halide. Does the better synthesis involve alkynide attack on bromoethane or on 2-bromopropane? Explain your reasoning.

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
59
Provide the major organic product of the reaction shown below. 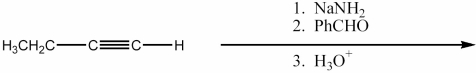
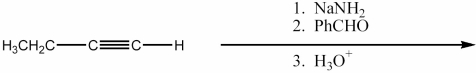

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
60
Provide the structure of the major organic product(s) in the reaction below. 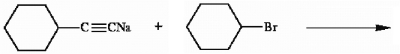


Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
61
What is the major organic product that results when 3-heptyne is treated with sodium metal in ammonia?
A) 2-heptyne
B) (Z)-2-heptene
C) (Z)-3-heptene
D) (E)-3-heptene
E) heptane
A) 2-heptyne
B) (Z)-2-heptene
C) (Z)-3-heptene
D) (E)-3-heptene
E) heptane

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
62
What is the major organic product that results when 3-heptyne is hydrogenated in the presence of Lindlar's catalyst?
A) 2-heptyne
B) (Z)-2-heptene
C) (Z)-3-heptene
D) (E)-3-heptene
E) heptane
A) 2-heptyne
B) (Z)-2-heptene
C) (Z)-3-heptene
D) (E)-3-heptene
E) heptane

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
63
Describe a sequence of reactions by which trans-pent-2-ene can be straightforwardly prepared from propyne.

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
64
Describe a sequence of reactions by which hept-1-yne can be straightforwardly prepared from hept-1-ene.

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
65
________ is produced when 1 equivalent of HBr is added to hex-1-yne in the presence of peroxides.
A) 2-Bromohex-1-ene
B) E-1-Bromohex-1-ene
C) Z-1-Bromohex-1-ene
D) A mixture of E and Z isomers of 1-bromohex-1-ene
E) E-2-Bromohex-2-ene
A) 2-Bromohex-1-ene
B) E-1-Bromohex-1-ene
C) Z-1-Bromohex-1-ene
D) A mixture of E and Z isomers of 1-bromohex-1-ene
E) E-2-Bromohex-2-ene

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
66
Why is a terminal alkyne favored when sodium amide (NaNH2) is used in an elimination reaction with 2,3-dichlorohexane?
A) The strong base deprotonates the terminal alkyne and removes it from the equilibrium.
B) The resonance favors the formation of the terminal rather than internal alkyne.
C) The positions of the Cl atoms induce the net formation of the terminal alkyne.
D) The terminal alkyne is more stable than the internal alkyne and is naturally the favored product.
E) The terminal alkyne is not favored in this reaction.
A) The strong base deprotonates the terminal alkyne and removes it from the equilibrium.
B) The resonance favors the formation of the terminal rather than internal alkyne.
C) The positions of the Cl atoms induce the net formation of the terminal alkyne.
D) The terminal alkyne is more stable than the internal alkyne and is naturally the favored product.
E) The terminal alkyne is not favored in this reaction.

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
67
Which of the following reagents will convert 1 mole of 3-methylpent-1-yne into 3-methylpentane?
A) 1 mole of Br2 in CCl4
B) 2 moles of Cl2 in CCl4
C) 2 moles of HCl
D) 2 moles H2, Ni and heat
E) 1 mole H2, Ni and heat
A) 1 mole of Br2 in CCl4
B) 2 moles of Cl2 in CCl4
C) 2 moles of HCl
D) 2 moles H2, Ni and heat
E) 1 mole H2, Ni and heat

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
68
Which of the following reagents should be used to convert hex-3-yne to (E)-hex-3-ene?
A) H2, Pt
B) Na, NH3
C) H2, Lindlar's catalyst
D) H2SO4, H2O
E) HgSO4, H2O
A) H2, Pt
B) Na, NH3
C) H2, Lindlar's catalyst
D) H2SO4, H2O
E) HgSO4, H2O

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
69
What is the major organic product that results when 1-heptyne is treated with 2 equivalents of HBr?
A) 2,3-dibromo-1-heptene
B) 2,3-dibromo-2-heptene
C) 1,2-dibromoheptane
D) 2,2-dibromoheptane
E) 1,1-dibromoheptane
A) 2,3-dibromo-1-heptene
B) 2,3-dibromo-2-heptene
C) 1,2-dibromoheptane
D) 2,2-dibromoheptane
E) 1,1-dibromoheptane

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
70
Which of the alkyne addition reactions below involve(s) an enol intermediate?
A) hydroboration/oxidation
B) treatment with HgSO4 in dilute H2SO4
C) hydrogenation
D) both A and B
E) none of the above
A) hydroboration/oxidation
B) treatment with HgSO4 in dilute H2SO4
C) hydrogenation
D) both A and B
E) none of the above

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
71
Name the compound which results when pent-2-yne is subjected to catalytic hydrogenation using a platinum catalyst.

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
72
Describe a sequence of reactions by which (E)-5-bromopent-2-ene can be straightforwardly prepared from propyne.

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
73
Which of the following describes an unsymmetrical addition reaction?
A) propyne with 1 mole H2, Ni and heat
B) propyne with 2 moles Cl2 in CCl4
C) propyne with 1 mole Br2 in CCl4
D) propyne with Na/NH3
E) propyne with 1 mole HBr
A) propyne with 1 mole H2, Ni and heat
B) propyne with 2 moles Cl2 in CCl4
C) propyne with 1 mole Br2 in CCl4
D) propyne with Na/NH3
E) propyne with 1 mole HBr

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
74
Provide the major organic product of the reaction below. 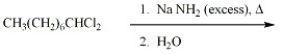
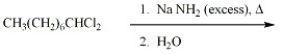

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
75
When 2,2-dibromobutane is heated at 200°C in the presence of molten KOH, what is the major organic product?
A) 1-bromobut-1-yne
B) 1-bromobut-2-yne
C) but-1-yne
D) but-2-yne
E) but-1-ene
A) 1-bromobut-1-yne
B) 1-bromobut-2-yne
C) but-1-yne
D) but-2-yne
E) but-1-ene

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
76
What is the major organic product that results when 3-heptyne is subjected to excess hydrogen and a platinum catalyst?
A) 2-heptyne
B) (Z)-2-heptene
C) (Z)-3-heptene
D) (E)-3-heptene
E) heptane
A) 2-heptyne
B) (Z)-2-heptene
C) (Z)-3-heptene
D) (E)-3-heptene
E) heptane

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
77
What class of organic product results when 1-heptyne is treated with a mixture of mercuric acetate in aqueous sulfuric acid?
A) aldehyde
B) ketone
C) diol
D) ether
E) carboxylic acid
A) aldehyde
B) ketone
C) diol
D) ether
E) carboxylic acid

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
78
Treatment of hex-2-yne with mercuric sulfate in dilute sulfuric acid yields a mixture of two ketones. Similar treatment of hex-3-yne produces a single ketone instead of a mixture. Explain.

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
79
Which of the following reagents should be used to convert hex-3-yne to (Z)-hex-3-ene?
A) H2, Pt
B) Na, NH3
C) H2, Lindlar's catalyst
D) H2SO4, H2O
E) HgSO4, H2O
A) H2, Pt
B) Na, NH3
C) H2, Lindlar's catalyst
D) H2SO4, H2O
E) HgSO4, H2O

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
80
A mixture of hept-1-yne, hept-2-yne, and hept-3-yne was hydrogenated in the presence of a platinum catalyst until hydrogen uptake ceased. If one assumes that the hydrogenation went to completion, how many seven-carbon hydrocarbons were produced?
A) 1
B) 2
C) 3
D) 6
E) 8
A) 1
B) 2
C) 3
D) 6
E) 8

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck



