Deck 5: Stereochemistry
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/128
Play
Full screen (f)
Deck 5: Stereochemistry
1
What term describes the structural relationship between (2R,3R,4S)-2,3,4-trichloroheptane and (2S,3S,5R)-2,3,5-trichloroheptane?
A) not isomers
B) constitutional isomers
C) enantiomers
D) diastereomers
E) conformers
A) not isomers
B) constitutional isomers
C) enantiomers
D) diastereomers
E) conformers
constitutional isomers
2
How many asymmetric carbon atoms are present in the following compound? 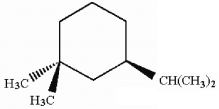
A) 0
B) 1
C) 2
D) 3
E) 4

A) 0
B) 1
C) 2
D) 3
E) 4
1
3
What is the structural relationship between the two molecule shown below? 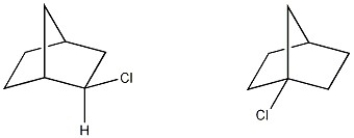
A) constitutional isomers
B) enantiomers
C) diastereomers
D) conformational isomers
E) not isomers

A) constitutional isomers
B) enantiomers
C) diastereomers
D) conformational isomers
E) not isomers
constitutional isomers
4
Which of the following terms best describes the pair of compounds shown: , or 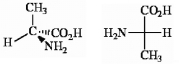
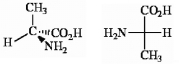

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
5
Which of the following describes -1,2-dimethylcyclopentane?
A) It is a meso compound.
B) It is achiral.
C) It contains two asymmetric carbons.
D) Its diastereomer is -1,2-dimethylcyclopentane.
E) It has an enantiomer.
A) It is a meso compound.
B) It is achiral.
C) It contains two asymmetric carbons.
D) Its diastereomer is -1,2-dimethylcyclopentane.
E) It has an enantiomer.

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
6
How many enantiomers are there of the molecule shown below? 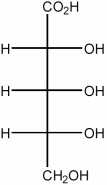
A) 0
B) 1
C) 2
D) 3
E) 6

A) 0
B) 1
C) 2
D) 3
E) 6

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
7
What term describes the structural relationship between (2R,3R,4S)-2,3,4-trichloroheptane and (2S,3S,4R)-2,3,4-trichloroheptane?
A) not isomers
B) constitutional isomers
C) enantiomers
D) diastereomers
E) conformers
A) not isomers
B) constitutional isomers
C) enantiomers
D) diastereomers
E) conformers

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
8
How many asymmetric carbon atoms are present in the molecule shown? 
A) 0
B) 1
C) 2
D) 3
E) 4

A) 0
B) 1
C) 2
D) 3
E) 4

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
9
Is the molecule shown below chiral or achiral?
(CH3)3CCH(CH3)2
(CH3)3CCH(CH3)2

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
10
How many asymmetric carbons are present in the compound below? 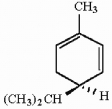


Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
11
How many asymmetric carbon atoms are present in the molecule shown? 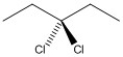
A) 0
B) 1
C) 2
D) 3
E) 4

A) 0
B) 1
C) 2
D) 3
E) 4

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
12
How many asymmetric carbons are present in the compound below? 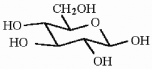


Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
13
________ are isomers which have the same bonding sequence but differ in the orientation of their atoms in space.

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
14
Which of the following terms best describes the pair of compounds shown: , or 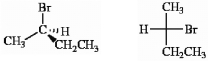
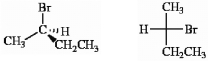

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
15
How many asymmetric carbon atoms are present in the molecule shown? 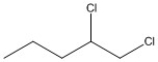
A) 0
B) 1
C) 2
D) 3
E) 4

A) 0
B) 1
C) 2
D) 3
E) 4

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
16
What term describes the structural relationship between -1,2-dimethylcyclopentane and trans-1,3-dimethylcyclopentane?
A) not isomers
B) constitutional isomers
C) enantiomers
D) diastereomers
E) conformers
A) not isomers
B) constitutional isomers
C) enantiomers
D) diastereomers
E) conformers

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
17
How many asymmetric carbons are present in the compound below? 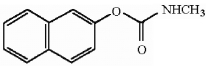


Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
18
How many asymmetric carbon atoms are present in the molecule shown? 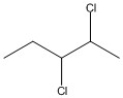
A) 0
B) 1
C) 2
D) 3
E) 4

A) 0
B) 1
C) 2
D) 3
E) 4

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
19
Which of the following terms best describes the pair of compounds shown: , or 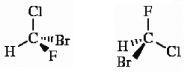
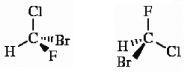

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
20
How many asymmetric carbon atoms are present in the following compound? 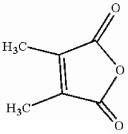
A) 0
B) 1
C) 2
D) 3
E) 4

A) 0
B) 1
C) 2
D) 3
E) 4

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
21
Is the molecule shown below chiral or achiral?
CH3CH2CH(CH3)CH2CH3
CH3CH2CH(CH3)CH2CH3

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
22
Is the molecule shown below chiral or achiral? 


Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
23
How many asymmetric carbons are present in the compound below? 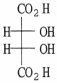


Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
24
If possible, draw the structure of the enantiomer of the molecule shown below. 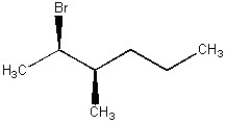


Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
25
Is the molecule shown below chiral or achiral? 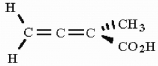


Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
26
Which of the following terms best describes the pair of compounds shown: , or 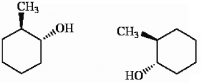
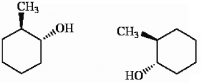

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
27
Which of the statements below correctly describes an achiral molecule?
A) The molecule has a nonsuperimposable mirror image.
B) The molecule exhibits optical activity when it interacts with plane-polarized light.
C) The molecule has an enantiomer.
D) The molecule might be a meso form.
E) None of the above
A) The molecule has a nonsuperimposable mirror image.
B) The molecule exhibits optical activity when it interacts with plane-polarized light.
C) The molecule has an enantiomer.
D) The molecule might be a meso form.
E) None of the above

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
28
Is the molecule shown below chiral or achiral? 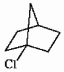


Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
29
Circle each chiral molecule among those shown below. 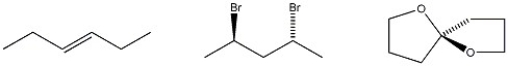


Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
30
Is the molecule shown below chiral or achiral? 


Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
31
Is the molecule shown below chiral or achiral? 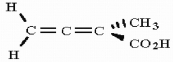
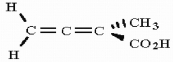

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
32
Is the molecule shown below chiral or achiral? 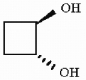


Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
33
Which of the following terms best describes the pair of compounds shown: , or 


Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
34
Circle all structures shown below that are chiral. 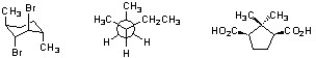


Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
35
Is the molecule shown below chiral or achiral? 


Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
36
Is the mirror image of the following molecule an enantiomer or is it superimposable with it? 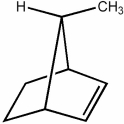


Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
37
Draw the enantiomer of the molecule shown below. 


Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
38
Circle each chiral molecule among those shown below. 


Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
39
Is the molecule shown below chiral or achiral? 


Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
40
How many asymmetric carbons are present in the compound below?
3-ethyl-2,2,4-trimethylpentane
3-ethyl-2,2,4-trimethylpentane

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
41
Draw the structure of (2R,3S)-2,3-dichloropentane. Take particular care to indicate three-dimensional stereochemical detail properly.

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
42
Label each asymmetric carbon in the compound below as R or S. 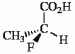


Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
43
Draw the Fischer projection of (S)-2-bromobutane.

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
44
Assign the proper configurational label, R or S, to the chiral carbon in the molecule shown below. 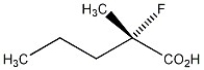


Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
45
Draw the structure of (S)-3-chloro-3-methylhexane. Take particular care to indicate stereochemistry properly.

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
46
Assign the proper configurational label, R or S, to each chiral carbon in the molecule below. 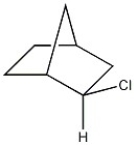


Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
47
Label each asymmetric carbon in the compound below as R or S. 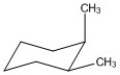


Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
48
Which of the following structures are achiral and meso? 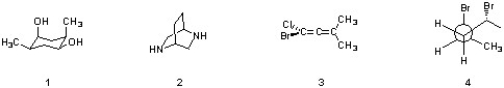
A) 1, 2, 3 & 4
B) 1 &2
C) 2 & 3
D) 1 & 4

A) 1, 2, 3 & 4
B) 1 &2
C) 2 & 3
D) 1 & 4

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
49
Label each asymmetric carbon in the compound below as R or S. 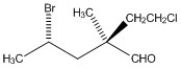


Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
50
Draw the structure of (1R, 2R)-1-bromo-2-chlorocyclobutane. Take particular care to indicate stereochemistry properly.

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
51
Label each asymmetric carbon in the molecule below as having the R or S configuration. 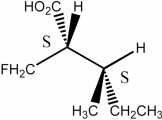


Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
52
How many asymmetric carbons are present in the compound below? 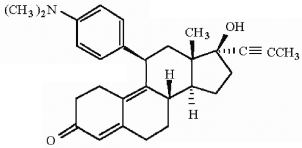


Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
53
Draw the structure of the enantiomer of (2S, 3R)-2,3-dichloropentane. Take particular care to indicate three-dimensional stereochemical detail properly.

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
54
Draw the structure of any diastereomer of (2S, 3R)-2,3-dichloropentane. Take particular care to indicate three-dimensional stereochemical detail properly.

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
55
Draw the structure of (S)-1-bromo-1-chloropropane. Take particular care to indicate three-dimensional stereochemical detail properly.

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
56
Draw the structure of (2R,3R)-2,3-dibromo-3-chloropentane.

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
57
Does the molecule shown below contain asymmetric carbon atoms? Is this molecule chiral? 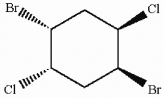


Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
58
Label each asymmetric carbon in the compound below as R or S. 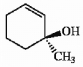


Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
59
Draw the structure of (1R, 2S, 3S)-1,2-dibromo-3-ethylcyclohexane. Take particular care to indicate stereochemistry properly.

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
60
Label each asymmetric carbon in the compound below as R or S. 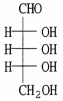


Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
61
If a mixture contains 75% of one compound and 25% of its enantiomer, what is the e.e. of the mixture?
A) 100
B) 75
C) 50
D) 25
E) 3
A) 100
B) 75
C) 50
D) 25
E) 3

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
62
Label each asymmetric carbon in the compound below as R or S. 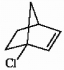


Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
63
Can one predict whether a compound with a single asymmetric carbon is dextro- or levorotatory based on the R/S assignment at this asymmetric carbon? Explain briefly.

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
64
Given that glucose has a specific rotation of +52.8°. Predict the concentration of a glucose aqueous solution contained in a 10 cm long polarimetry tube if a rotation of +15.8° was observed.
A) 0.299 g/mL
B) 0299 g/mL
C) 3.34 g/mL
D) 334 g/mL
A) 0.299 g/mL
B) 0299 g/mL
C) 3.34 g/mL
D) 334 g/mL

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
65
A mixture of equal amounts of two enantiomers ________.
A) is called a racemic mixture
B) is optically inactive
C) implies that the enantiomers are meso forms
D) both A and B
E) none of the above
A) is called a racemic mixture
B) is optically inactive
C) implies that the enantiomers are meso forms
D) both A and B
E) none of the above

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
66
Which of the following statements correctly pertains to a pair of enantiomers?
A) They rotate the plane of polarized light by exactly the same amount and in opposite directions.
B) They rotate the plane of polarized light by differing amounts and in opposite directions.
C) They rotate the plane of polarized light by differing amounts and in the same direction.
D) The have different melting points.
E) They have the same melting point, but they have different boiling points.
A) They rotate the plane of polarized light by exactly the same amount and in opposite directions.
B) They rotate the plane of polarized light by differing amounts and in opposite directions.
C) They rotate the plane of polarized light by differing amounts and in the same direction.
D) The have different melting points.
E) They have the same melting point, but they have different boiling points.

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
67
Which of the following statements is (are) true for the compound (R)-2-butanol?
A) This compound is chiral.
B) This compound is optically active.
C) This compound has an enantiomer.
D) all of the above
E) none of the above
A) This compound is chiral.
B) This compound is optically active.
C) This compound has an enantiomer.
D) all of the above
E) none of the above

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
68
A mixture of two enantiomers with a composition of 65.0% R has an observed rotation of -25.3° in a ![<strong>A mixture of two enantiomers with a composition of 65.0% R has an observed rotation of -25.3° in a polarimeter tube. If the mixture has a concentration of 2.038 g/mL at 25°C, what is the predicted [α]<sup>25</sup>D of an optically pure sample of the S enantiomer?</strong> A) -25.3° B) -53.5° C) +12.4° D) +41.3° E) +53.5°](https://d2lvgg3v3hfg70.cloudfront.net/TB6198/11eab45f_cd7d_6078_acdb_a5eba3fbfe3e_TB6198_00.jpg) polarimeter tube. If the mixture has a concentration of 2.038 g/mL at 25°C, what is the predicted [α]25D of an optically pure sample of the S enantiomer?
polarimeter tube. If the mixture has a concentration of 2.038 g/mL at 25°C, what is the predicted [α]25D of an optically pure sample of the S enantiomer?
A) -25.3°
B) -53.5°
C) +12.4°
D) +41.3°
E) +53.5°
![<strong>A mixture of two enantiomers with a composition of 65.0% R has an observed rotation of -25.3° in a polarimeter tube. If the mixture has a concentration of 2.038 g/mL at 25°C, what is the predicted [α]<sup>25</sup>D of an optically pure sample of the S enantiomer?</strong> A) -25.3° B) -53.5° C) +12.4° D) +41.3° E) +53.5°](https://d2lvgg3v3hfg70.cloudfront.net/TB6198/11eab45f_cd7d_6078_acdb_a5eba3fbfe3e_TB6198_00.jpg) polarimeter tube. If the mixture has a concentration of 2.038 g/mL at 25°C, what is the predicted [α]25D of an optically pure sample of the S enantiomer?
polarimeter tube. If the mixture has a concentration of 2.038 g/mL at 25°C, what is the predicted [α]25D of an optically pure sample of the S enantiomer?A) -25.3°
B) -53.5°
C) +12.4°
D) +41.3°
E) +53.5°

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
69
A student measured the optical activity of an unknown sugar at two different concentrations. The results of his measurements are shown below. Given that the sample cell had a path length of 10.0 cm, calculate the specific rotation for the unknown sugar. (Hint: Consider each measurement of plane polarized light has a true reading and a "ghost" reading 180° from the true reading). 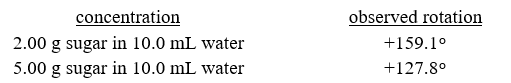
A) -10.5∘
B) +25.6∘
C) +79.5∘
D) -105∘
E) +256∘
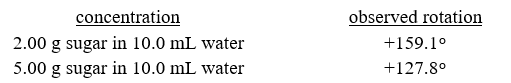
A) -10.5∘
B) +25.6∘
C) +79.5∘
D) -105∘
E) +256∘

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
70
Compounds that rotate the plane of polarized light clockwise are called ________.

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
71
Would a 50:50 mixture of (2R,3R)-2,3-dibromobutane and (2R,3S)-2,3-dibromobutane be optically active? Explain.

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
72
Which of the following configurations corresponds to the structure below? 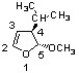
A) (4R, 5R)
B) (4R, 5S)
C) (4S, 5R)
D) (4S, 5S)

A) (4R, 5R)
B) (4R, 5S)
C) (4S, 5R)
D) (4S, 5S)

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
73
If (S)-glyceraldehyde has a specific rotation of -8.7°, what is the specific rotation of (R)-glyceraldehyde?
A) 0.0°
B) -8.7°
C) +8.7°
D) cannot be determined from the information given
A) 0.0°
B) -8.7°
C) +8.7°
D) cannot be determined from the information given

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
74
Captopril is used to treat high blood pressure and congestive heart failure. Label the chiral centers as R or S. 


Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
75
For the structure shown below, draw the stereoisomer having a configuration of (1R,3S,4S) in a perspective structure. 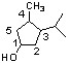


Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
76
Predict the specific rotation of the compound shown. 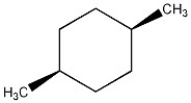
A) It is impossible to predict; it must be determined experimentally.
B) Because both asymmetric centers are R, the compound is dextrorotatory.
C) Because both asymmetric centers are S, the compound is levorotatory.
D) Zero; the compound is achiral.
E) Because this compound represents a racemic mixture, the compound is dextrorotatory.

A) It is impossible to predict; it must be determined experimentally.
B) Because both asymmetric centers are R, the compound is dextrorotatory.
C) Because both asymmetric centers are S, the compound is levorotatory.
D) Zero; the compound is achiral.
E) Because this compound represents a racemic mixture, the compound is dextrorotatory.

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
77
Label each asymmetric carbon in the compound below as R or S. 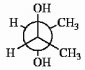


Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
78
A newly isolated natural product was shown to be optically active. If a solution of 2.0 g in 10 mL of ethanol in a 50 cm tube gives a rotation of +2.57°, what is the specific rotation of this natural product?

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
79
Calculate the e.e. of a mixture containing 8.0 g of (-)-glyceraldehyde and 2.0 g of (+)-glyceraldehyde.

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
80
Phantasmidine, shown below, is found in poisonous frog skin and has analgesic properties (J. Nat. Prod. 331). Assign each chiral center as having either R or S configuration. 


Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck



