Deck 13: Nuclear Magnetic Resonance Spectroscopy
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/130
Play
Full screen (f)
Deck 13: Nuclear Magnetic Resonance Spectroscopy
1
The protons marked Ha and Hb in the molecule below are ________. 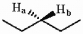
A) chemically equivalent or homotopic
B) enantiotopic
C) diastereotopic
D) endotopic
E) none of the above

A) chemically equivalent or homotopic
B) enantiotopic
C) diastereotopic
D) endotopic
E) none of the above
chemically equivalent or homotopic
2
What three-word term is abbreviated NMR?
nuclear magnetic resonance
3
Predict the number of signals expected (disregarding splitting) in the 1H NMR spectrum of the compound shown below. 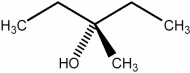

4
4
Which of the following reasons best explains why aromatic protons have a larger chemical shift than protons one carbon removed from a halogen?
A) An aromatic ring is more electron withdrawing than a halogen.
B) A halogen is more electron withdrawing than an aromatic ring.
C) Electron movement induces a magnetic field opposing the external field.
D) Electron movement induces a magnetic field reinforcing the external field.
A) An aromatic ring is more electron withdrawing than a halogen.
B) A halogen is more electron withdrawing than an aromatic ring.
C) Electron movement induces a magnetic field opposing the external field.
D) Electron movement induces a magnetic field reinforcing the external field.

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
5
On a 90 MHz spectrometer, calculate the frequency at which a proton absorbs if it appears at 4.20 ppm.

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
6
1H nuclei located near electronegative atoms tend to be ________ relative to 1H nuclei which are not.
A) shielded
B) deshielded
C) resonanced
D) split
E) none of the above
A) shielded
B) deshielded
C) resonanced
D) split
E) none of the above

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
7
How many nuclear spin states are allowed for the 1H nucleus?
A) 1
B) 2
C) 3
D) 4
E) 10
A) 1
B) 2
C) 3
D) 4
E) 10

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
8
If a molecule contains 4 elements of unsaturation and signals in the 1H NMR spectrum between δ 7.0 and 8.0 ppm, what structural group is likely to be present?
A) a carbonyl group
B) an aromatic ring
C) a hydroxyl group
D) a cyclohexyl ring
E) a carbon-carbon triple bond
A) a carbonyl group
B) an aromatic ring
C) a hydroxyl group
D) a cyclohexyl ring
E) a carbon-carbon triple bond

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
9
A nucleus with an ________ atomic number or an ________ mass number has a nuclear spin that can be observed by the NMR spectrometer.
A) even, odd
B) odd, even
C) odd, odd
D) even, even
A) even, odd
B) odd, even
C) odd, odd
D) even, even

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
10
In the presence of an external magnetic field the approximate ratio of 1H nuclei with α spins compared to β spins is respectively: ________.
A) 90:10
B) 25:75
C) 50:50
D) 75:25
A) 90:10
B) 25:75
C) 50:50
D) 75:25

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
11
Predict the number of signals expected (disregarding splitting) in the 1H NMR spectrum of the compound shown below. 


Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
12
Calculate the magnetic field that corresponds to the proton resonance frequency of 300.00 MHz. The gyromagnetic ratio of the 1H nucleus is 26,753 s-1 gauss-1.

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
13
Predict the number of signals expected (disregarding splitting) in the 1H spectrum of m-xylene (1,3-dimethylbenzene).

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
14
The energy difference between the allowed spin states for an 1H nucleus is ________ the strength of the external magnetic field in which it is placed.
A) independent of
B) directly proportional to
C) inversely proportional to
D) exponentially related to
E) logarithmically related to
A) independent of
B) directly proportional to
C) inversely proportional to
D) exponentially related to
E) logarithmically related to

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
15
Predict the number of signals expected (disregarding splitting) in the 1H spectrum of dibutyl ether.

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
16
Predict the number of signals expected (disregarding splitting) in the 1H spectrum of o-chlorophenol (2-chlorophenol).

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
17
Using a 60-MHz spectrometer, the protons in dichloromethane appear at 5.30 ppm. When the same sample is placed in a 100-MHz instrument, where does the signal appear?
A) 8.33
B) 5.30
C) 3.18
D) cannot be determined from information given
A) 8.33
B) 5.30
C) 3.18
D) cannot be determined from information given

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
18
Electromagnetic radiation in the ________ region is used in 1H NMR spectroscopy.
A) radio wave
B) ultraviolet
C) infrared
D) microwave
E) X-ray
A) radio wave
B) ultraviolet
C) infrared
D) microwave
E) X-ray

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
19
Which of the following functional groups has the most deshielded proton and would be predicted to show an NMR signal that is the farthest downfield?
A) benzylic - H
B) allylic - H
C) aldehyde - H
D) aromatic - H
E) acetylenic - H
A) benzylic - H
B) allylic - H
C) aldehyde - H
D) aromatic - H
E) acetylenic - H

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
20
________ is commonly used as an internal reference in NMR spectroscopy; its signal is assigned d = 0 in 1H and 13C NMR spectroscopy.

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
21
A compound (C5H8O) shows IR absorptions at 3600 and 3300 cm-1. Its 1H NMR spectrum contained singlets at 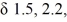 and 2.9 (broad) (ppm) in a ratio of 6:1:1. Name this compound.
and 2.9 (broad) (ppm) in a ratio of 6:1:1. Name this compound.
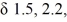 and 2.9 (broad) (ppm) in a ratio of 6:1:1. Name this compound.
and 2.9 (broad) (ppm) in a ratio of 6:1:1. Name this compound.
Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
22
What 1H NMR spectral data is expected for the compound shown? 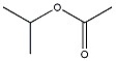
A) 3.8 (1H, septet), 2.1 (3H, s), 1.0 (6H, d)
B) 3.8 (1H, septet), 3.3 (3H, s), 1.0 (6H, d)
C) 3.3 (3H, s), 2.6 (3H, septet), 1.0 (6H, d)
D) 2.6 (1H, septet), 2.1 (3H, s), 1.0 (6H, d)

A) 3.8 (1H, septet), 2.1 (3H, s), 1.0 (6H, d)
B) 3.8 (1H, septet), 3.3 (3H, s), 1.0 (6H, d)
C) 3.3 (3H, s), 2.6 (3H, septet), 1.0 (6H, d)
D) 2.6 (1H, septet), 2.1 (3H, s), 1.0 (6H, d)

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
23
What compound exhibits only two signals in its 1H NMR spectrum, a triplet and a quintet?
A) BrCH2CH2CH2Br
B) BrCH2CH2CH2Cl
C) (CH3)2CHCH(CH3)2
D) CH3CH2CH2CH3
E) (CH3)2CHOCH(CH3)2
A) BrCH2CH2CH2Br
B) BrCH2CH2CH2Cl
C) (CH3)2CHCH(CH3)2
D) CH3CH2CH2CH3
E) (CH3)2CHOCH(CH3)2

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
24
The protons marked Ha and Hb in the molecule below are ________. 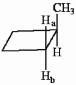
A) chemically equivalent
B) enantiotopic
C) diastereotopic
D) endotopic
E) none of the above

A) chemically equivalent
B) enantiotopic
C) diastereotopic
D) endotopic
E) none of the above

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
25
What is the relationships between Ha and Hb in the following structure? 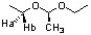
A) homeotopic
B) enantiotopic
C) diastereotopic
D) none of the previous

A) homeotopic
B) enantiotopic
C) diastereotopic
D) none of the previous

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
26
Use the structure below to state the relationship between the indicated protons as equivalent, enantiotopic, diastereotopic, or unrelated. 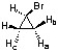 Ha & Hb:
Ha & Hb:
Ha & Hc:
Hb & Hc:
 Ha & Hb:
Ha & Hb:Ha & Hc:
Hb & Hc:

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
27
What is the relative area of each peak in a quartet spin-spin splitting pattern?
A) 1:2:1
B) 1:2:2:1
C) 1:1:1:1
D) 1:4:4:1
E) 1:3:3:1
A) 1:2:1
B) 1:2:2:1
C) 1:1:1:1
D) 1:4:4:1
E) 1:3:3:1

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
28
The following splitting pattern represents one of the vinyl protons of styrene. Identify which proton is represented and list all the coupling constants (J values) for the splitting pattern. 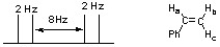


Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
29
In the 1H NMR spectrum of bromoethane the methylene group is split into a quartet by the α and β nuclear spins of the protons on the neighboring methyl group. If the external magnetic field, B∘, directs upward, which sequence of nuclear spins contributes to the second farthest peak down field within the spin-spin splitting pattern?
A) ↑↓↓
B) ↑↓↑
C) ↑↑↑
D) ↓↓↓
E) ↑↑↓↓
A) ↑↓↓
B) ↑↓↑
C) ↑↑↑
D) ↓↓↓
E) ↑↑↓↓

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
30
Describe the number of signals and their splitting in the 1H NMR spectrum of (CH3)2CHOCH3.
A) 3 signals: 2 doublets and a septet
B) 2 signals: a doublet and a septet
C) 3 signals: a doublet, a quartet, and a septet
D) 4 signals: 2 doublets, a singlet, and a septet
E) 3 signals: a singlet, a doublet, and a septet
A) 3 signals: 2 doublets and a septet
B) 2 signals: a doublet and a septet
C) 3 signals: a doublet, a quartet, and a septet
D) 4 signals: 2 doublets, a singlet, and a septet
E) 3 signals: a singlet, a doublet, and a septet

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
31
Predict the number of signals expected, their splitting, and their relative area in the 1H NMR spectrum of 1,2-dichloroethane (ClCH2CH2Cl).

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
32
Predict the number of signals expected (disregarding splitting) in the 1H spectrum of 1,1-dimethylcyclobutane.

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
33
The protons marked Ha and Hb in the molecule below are ________. 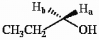
A) chemically equivalent
B) enantiotopic
C) diastereotopic
D) endotopic
E) none of the above
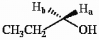
A) chemically equivalent
B) enantiotopic
C) diastereotopic
D) endotopic
E) none of the above

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
34
Why is carbon-hydrogen splitting not a major part of 1H NMR spectra?

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
35
A peak split into a doublet of triplets gave the following measurements in its splitting
pattern. State the coupling constants (J values) for both the doublet and the triplet.
pattern. State the coupling constants (J values) for both the doublet and the triplet.


Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
36
Label the different 1H environments in the structure below a, b, c.... Then complete the table for each different type of proton. The first one is done for you as an example. (Note: there may be fewer than 7 different proton environments) 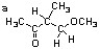 a b c d e f g
a b c d e f g
(+/- 0.5 ppm) 1.9
s
 a b c d e f g
a b c d e f g(+/- 0.5 ppm) 1.9
s

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
37
What 1H NMR spectral data is expected for the compound shown? 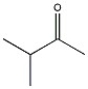
A) 3.8 (1H, septet), 2.1 (3H, s), 1.0 (6H, d)
B) 3.8 (1H, septet), 3.3 (3H, s), 1.0 (6H, d)
C) 3.3 (3H, s), 2.6 (3H, septet), 1.0 (6H, d)
D) 2.6 (1H, septet), 2.1 (3H, s), 1.0 (6H, d)

A) 3.8 (1H, septet), 2.1 (3H, s), 1.0 (6H, d)
B) 3.8 (1H, septet), 3.3 (3H, s), 1.0 (6H, d)
C) 3.3 (3H, s), 2.6 (3H, septet), 1.0 (6H, d)
D) 2.6 (1H, septet), 2.1 (3H, s), 1.0 (6H, d)

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
38
Predict the number of signals expected, their splitting, and their relative area in the 1H NMR spectrum of (CH3)3CCHO.

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
39
Predict the number of signals expected, their splitting, and their relative area in the 1H NMR spectrum of 2-methylpropane (isobutane).

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
40
Predict the number of signals expected, their splitting, and their relative area in the 1H NMR spectrum of CH3CH2OCH3.

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
41
Any process faster than ________ will be recorded as an average by NMR spectroscopy.
A) 0.0005 seconds
B) 0.1 seconds
C) 0.01 seconds
D) 0.001 seconds
E) 1 minute
A) 0.0005 seconds
B) 0.1 seconds
C) 0.01 seconds
D) 0.001 seconds
E) 1 minute

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
42
Predict the number of signals expected in the proton spin decoupled 13C spectrum of cyclopentane.

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
43
The 1H NMR spectrum of ethanol is acquired and the hydroxyl signal appears as a singlet instead of a triplet. Offer an explanation.

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
44
What type of carbon environment does not generate a signal in the DEPT-90 spectrum and gives a negative signal in the DEPT-135 spectrum?
A) quaternary
B) methine
C) methylene
D) methyl
E) carbonyl
A) quaternary
B) methine
C) methylene
D) methyl
E) carbonyl

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
45
What is the approximate chemical shift of an alkynyl carbon in 13C NMR spectroscopy?
A) 10 ppm
B) 30 ppm
C) 70 ppm
D) 120 ppm
E) 200 ppm
A) 10 ppm
B) 30 ppm
C) 70 ppm
D) 120 ppm
E) 200 ppm

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
46
What type of carbon environment does not generate a signal in the DEPT-90 spectrum and gives a positive peak in the DEPT-135 spectrum?
A) quaternary
B) methine
C) methylene
D) methyl
E) carbonyl
A) quaternary
B) methine
C) methylene
D) methyl
E) carbonyl

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
47
What compound exhibits three singlets in its proton spin decoupled 13C NMR spectrum?
A) BrCH2CH2CH2Br
B) BrCH2CH2CH2Cl
C) (CH3)2CHCH(CH3)2
D) CH3CH2CH2CH3
E) (CH3)2CHOCH(CH3)2
A) BrCH2CH2CH2Br
B) BrCH2CH2CH2Cl
C) (CH3)2CHCH(CH3)2
D) CH3CH2CH2CH3
E) (CH3)2CHOCH(CH3)2

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
48
How many peaks appear in the proton spin decoupled 13 C NMR spectrum of the compound below? 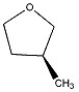
A) 1
B) 2
C) 3
D) 4
E) 5

A) 1
B) 2
C) 3
D) 4
E) 5

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
49
You have a sample and its 1H NMR spectrum. You know your sample contains O atoms but not N atoms, and you suspect that your sample may be an alcohol. What common spectroscopic technique might you use to confirm your suspicion?

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
50
Explain the difference between off-resonance decoupling and broadband decoupling and how each affects the C-13 NMR spectrum. In your discussion include a short description of how a chemist interprets the different spectra.

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
51
An NMR spectrometer that operates at a frequency of 60 MHz for 13C NMR spectra, operates at what frequency for 1H NMR spectra?
A) 15 MHz
B) 30 MHz
C) 60 MHz
D) 120 MHz
E) 240 MHz
A) 15 MHz
B) 30 MHz
C) 60 MHz
D) 120 MHz
E) 240 MHz

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
52
Predict the number of signals expected in the proton spin decoupled 13C NMR spectrum of the compound shown below. 


Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
53
How might the proton spectrum of ultrapure dimethylamine, (CH3)2NH, differ from the spectrum of this compound to which D2O has been added?

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
54
What multiplicities are observed in the off-resonance decoupled 13C spectrum of 2,3-dimethyl-but-2-ene?

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
55
How many peaks appear in the proton spin decoupled 13 C NMR spectrum of the compound below? 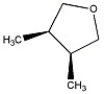
A) 2
B) 3
C) 4
D) 5
E) 6

A) 2
B) 3
C) 4
D) 5
E) 6

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
56
Give one reason why 13C NMR is less sensitive than 1H NMR.

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
57
Why is carbon-carbon splitting typically not seen in 13C NMR spectra?

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
58
If a signal is observed in the 170 to 200 ppm range in a C-13 NMR spectrum, what is the most likely type of functional group associated with that carbon atom?
A) carbonyl carbon
B) allylic carbon
C) aromatic carbon
D) carbon/carbon triple bond
E) carbon/carbon double bond
A) carbonyl carbon
B) allylic carbon
C) aromatic carbon
D) carbon/carbon triple bond
E) carbon/carbon double bond

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
59
Which compound generates positive peaks for the carbonyl in both its DEPT-90 and DEPT-135 spectra?
A) CH3CH2CHO
B) CH3CH2COCH3
C) CH3CO2CH2CH3
D) CH3CH2CONH2
E) H2CO
A) CH3CH2CHO
B) CH3CH2COCH3
C) CH3CO2CH2CH3
D) CH3CH2CONH2
E) H2CO

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
60
The chair form of cyclohexane has protons in two distinct environments, axial and equatorial. When the proton NMR of cyclohexane is run on a 100-MHz instrument at 23°C, only one signal for the compound is observed. Explain this apparent contradiction.

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
61
How many peaks appear in the proton spin decoupled 13C NMR spectrum of the compound below? 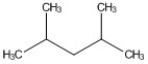


Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
62
Predict the number of distinct quartets expected in the off-resonance decoupled 13C NMR spectrum of the compound shown below. 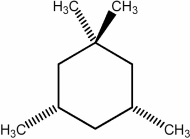


Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
63
How many peaks appear in the proton spin decoupled 13C NMR spectrum of the compound below? 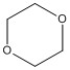


Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
64
How many peaks appear in the proton spin decoupled 13 C NMR spectrum of the compound below? 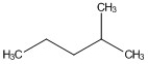


Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
65
How many peaks appear in the proton spin decoupled 13C NMR spectrum of the compound below? 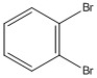


Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
66
How many peaks appear in the proton spin decoupled 13C NMR spectrum of the compound below? 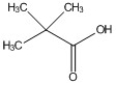


Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
67
How many peaks appear in the proton spin decoupled 13C NMR spectrum of the compound below? 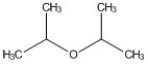


Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
68
Predict the number of signals expected in the proton spin decoupled 13C NMR spectrum of o-diethylbenzene (1,2-diethylbenzene).

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
69
Predict the number of signals expected in the proton spin decoupled 13C spectrum of p-dibromobenzene (1,4-dibromobenzene).

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
70
Predict the number of signals expected in the proton spin decoupled 13C spectrum of m-dichlorobenzene (1,3-dichlorobenzene).

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
71
Predict the number of signals expected in the proton spin decoupled 13C NMR spectrum of the compound shown below. 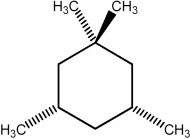


Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
72
How many peaks appear in the proton spin decoupled 13C NMR spectrum of the compound below? 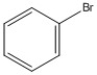


Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
73
Predict the number of distinct quartets expected in the off-resonance decoupled 13C NMR spectrum of the compound shown below. 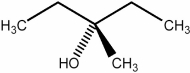


Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
74
Predict the number of signals expected in the proton spin decoupled 13C NMR spectrum of p-diethylbenzene (1,4-diethylbenzene).

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
75
Predict the number of signals expected in the proton spin decoupled 13C spectrum of 3-hexanone, CH3CH2COCH2CH2CH3.

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
76
Predict the number of distinct quartets expected in the off-resonance decoupled 13C NMR spectrum of the compound shown below. 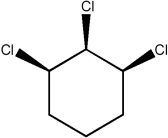


Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
77
Predict the number of distinct quartets expected in the off-resonance decoupled 13C NMR spectrum of the compound shown below. 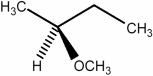


Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
78
How many peaks appear in the proton spin decoupled 13C NMR spectrum of the compound below? 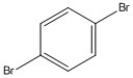


Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
79
Predict the number of signals expected in the proton spin decoupled 13C NMR spectrum of the compound shown below. 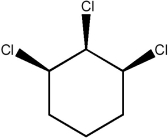


Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
80
Predict the number of signals expected in the proton spin decoupled 13C NMR spectrum of the compound shown below. 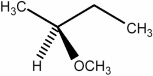


Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck



