Deck 13: Nuclear Magnetic Resonance Spectroscopy
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/130
Play
Full screen (f)
Deck 13: Nuclear Magnetic Resonance Spectroscopy
1
If a molecule contains 4 elements of unsaturation and signals in the 1H NMR spectrum between δ 7.0 and 8.0 ppm, what structural group is likely to be present?
A) a carbonyl group
B) an aromatic ring
C) a hydroxyl group
D) a cyclohexyl ring
E) a carbon-carbon triple bond
A) a carbonyl group
B) an aromatic ring
C) a hydroxyl group
D) a cyclohexyl ring
E) a carbon-carbon triple bond
an aromatic ring
2
Predict the number of signals expected (disregarding splitting) in the 1H spectrum of m-xylene (1,3-dimethylbenzene).
4
3
What are the relative integrations for the 1H NMR signals for the following compound? 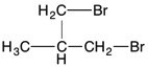
A) 3:1:2:2
B) 3:3:2
C) 3:1:4
D) 5:3

A) 3:1:2:2
B) 3:3:2
C) 3:1:4
D) 5:3
3:1:4
4
How many nuclear spin states are allowed for the 1H nucleus?
A) 1
B) 2
C) 3
D) 4
E) 10
A) 1
B) 2
C) 3
D) 4
E) 10

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
5
On a 90 MHz spectrometer, calculate the frequency at which a proton absorbs if it appears at 4.20 ppm.

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
6
The protons marked Ha and Hb in the molecule below are ________. 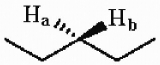
A) chemically equivalent or homotopic
B) enantiotopic
C) diastereotopic
D) endotopic
E) none of the above
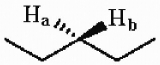
A) chemically equivalent or homotopic
B) enantiotopic
C) diastereotopic
D) endotopic
E) none of the above

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
7
________ is commonly used as an internal reference in NMR spectroscopy; its signal is assigned δ = 0 in 1H and 13C NMR spectroscopy.

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
8
Which of the following functional groups has the most deshielded proton and would be predicted to show an NMR signal that is the farthest downfield?
A) benzylic - H
B) allylic - H
C) aldehyde - H
D) aromatic - H
E) acetylenic - H
A) benzylic - H
B) allylic - H
C) aldehyde - H
D) aromatic - H
E) acetylenic - H

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
9
What information does the intensity (integration) of a signal in the 1H NMR spectrum give you?
A) how many different types of protons are present
B) the electronic structure of the molecule close to each type of proton
C) how many protons of each type are present
D) how many neighboring protons are present
A) how many different types of protons are present
B) the electronic structure of the molecule close to each type of proton
C) how many protons of each type are present
D) how many neighboring protons are present

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
10
Calculate the magnetic field that corresponds to the proton resonance frequency of 300.00 MHz. The gyromagnetic ratio of the 1H nucleus is 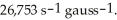
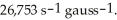

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
11
Predict the number of signals expected (disregarding splitting) in the 1H spectrum of o-chlorophenol (2-chlorophenol).

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
12
The energy difference between the allowed spin states for an 1H nucleus is ________ the strength of the external magnetic field in which it is placed.
A) independent of
B) directly proportional to
C) inversely proportional to
D) exponentially related to
E) logarithmically related to
A) independent of
B) directly proportional to
C) inversely proportional to
D) exponentially related to
E) logarithmically related to

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
13
Which of the following reasons best explains why aromatic protons have a larger chemical shift than protons one carbon removed from a halogen?
A) An aromatic ring is more electron withdrawing than a halogen.
B) A halogen is more electron withdrawing than an aromatic ring.
C) Electron movement induces a magnetic field opposing the external field.
D) Electron movement induces a magnetic field reinforcing the external field.
A) An aromatic ring is more electron withdrawing than a halogen.
B) A halogen is more electron withdrawing than an aromatic ring.
C) Electron movement induces a magnetic field opposing the external field.
D) Electron movement induces a magnetic field reinforcing the external field.

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
14
What three-word term is abbreviated NMR?

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
15
Predict the number of signals expected (disregarding splitting) in the 1H NMR spectrum of the compound shown below. 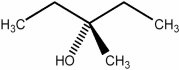


Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
16
Using a 60 MHz spectrometer, the protons in dichloromethane appear at 5.30 ppm. When the same sample is placed in a 100 MHz instrument, where does the signal appear?
A) 8.33
B) 5.30
C) 3.18
D) cannot be determined from information given
A) 8.33
B) 5.30
C) 3.18
D) cannot be determined from information given

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
17
1H nuclei located near electronegative atoms tend to be ________ relative to 1H nuclei which are not.
A) shielded
B) deshielded
C) resonanced
D) split
E) none of the above
A) shielded
B) deshielded
C) resonanced
D) split
E) none of the above

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
18
Predict the number of signals expected (disregarding splitting) in the 1H spectrum of dibutyl ether.

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
19
A nucleus with an ________ atomic number or an ________ mass number has a nuclear spin that can be observed by the NMR spectrometer.
A) even; odd
B) odd; even
C) odd; odd
D) even; even
A) even; odd
B) odd; even
C) odd; odd
D) even; even

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
20
Predict the number of signals expected (disregarding splitting) in the 1H NMR spectrum of the compound shown below. 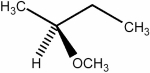


Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
21
What 1H NMR spectral data is expected for the compound shown? 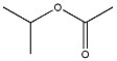
A) 3.8 (1H, septet), 2.1 (3H, s), 1.0 (6H, d)
B) 3.8 (1H, septet), 3.3 (3H, s), 1.0 (6H, d)
C) 3.3 (3H, s), 2.6 (3H, septet), 1.0 (6H, d)
D) 2.6 (1H, septet), 2.1 (3H, s), 1.0 (6H, d)

A) 3.8 (1H, septet), 2.1 (3H, s), 1.0 (6H, d)
B) 3.8 (1H, septet), 3.3 (3H, s), 1.0 (6H, d)
C) 3.3 (3H, s), 2.6 (3H, septet), 1.0 (6H, d)
D) 2.6 (1H, septet), 2.1 (3H, s), 1.0 (6H, d)

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
22
Predict the number of signals expected, their splitting, and their relative area in the 1H NMR spectrum of (CH3)3CCHO.

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
23
The protons labeled Ha in the structure below generally show up as a triplet, even through there are three neighboring protons. The coupling constant is approximately 7 Hz. What does this suggest about the size of the coupling constant for the aldehydic proton? 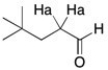


Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
24
Use the structure below to state the relationship between the indicated protons as equivalent, enantiotopic, diastereotopic, or unrelated. 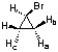
Ha & Hb:
Ha & Hc:
Hb & Hc:

Ha & Hb:
Ha & Hc:
Hb & Hc:

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
25
The protons marked Ha and Hb in the molecule below are ________. 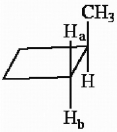
A) chemically equivalent
B) enantiotopic
C) diastereotopic
D) endotopic
E) none of the above

A) chemically equivalent
B) enantiotopic
C) diastereotopic
D) endotopic
E) none of the above

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
26
Predict the number of signals expected (disregarding splitting) in the 1H spectrum of 1,1-dimethylcyclobutane.

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
27
A peak split into a doublet of triplets gave the following measurements in its splitting pattern. State the coupling constants (J values) for both the doublet and the triplet. 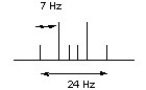


Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
28
The following splitting pattern represents one of the vinyl protons of styrene. Identify which proton is represented and list all the coupling constants (J values) for the splitting pattern. 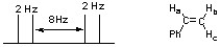


Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
29
A compound (C5H8O) shows IR absorptions at 3600 and 3300 cm-1. Its 1H NMR spectrum contained singlets at 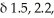
and 2.9 (broad) (ppm) in a ratio of 6:1:1. Name this compound.
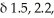
and 2.9 (broad) (ppm) in a ratio of 6:1:1. Name this compound.

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
30
Label the different 1H environments in the structure below a, b, c.... Then complete the table for each different type of proton. The first one is done for you as an example. (Note: there may be fewer than 7 different proton environments) 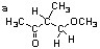
environments a b c d e f g
chemical shift
(+/- 0.5 ppm) 1.9
spin-spin splitting s

environments a b c d e f g
chemical shift
(+/- 0.5 ppm) 1.9
spin-spin splitting s

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
31
Predict the number of signals expected, their splitting, and their relative area in the 1H NMR spectrum of 2-methylpropane (isobutane).

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
32
Describe the number of signals and their splitting in the 1H NMR spectrum of (CH3)2CHOCH3.
A) 3 signals: 2 doublets and a septet
B) 2 signals: a doublet and a septet
C) 3 signals: a doublet, a quartet, and a septet
D) 4 signals: 2 doublets, a singlet, and a septet
E) 3 signals: a singlet, a doublet, and a septet
A) 3 signals: 2 doublets and a septet
B) 2 signals: a doublet and a septet
C) 3 signals: a doublet, a quartet, and a septet
D) 4 signals: 2 doublets, a singlet, and a septet
E) 3 signals: a singlet, a doublet, and a septet

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
33
What is the relative area of each peak in a quartet spin-spin splitting pattern?
A) 1:2:1
B) 1:2:2:1
C) 1:1:1:1
D) 1:4:4:1
E) 1:3:3:1
A) 1:2:1
B) 1:2:2:1
C) 1:1:1:1
D) 1:4:4:1
E) 1:3:3:1

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
34
What is the expected multiplicity and relative ratio of the area of the peak for the protons labeled Ha? 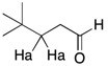
A) singlet with relative area of 1
B) triplet with relative area of 1:1:1
C) triplet with relative area of 1:3:1
D) multiplet with relative area of 1:6:15:20:15:6:1

A) singlet with relative area of 1
B) triplet with relative area of 1:1:1
C) triplet with relative area of 1:3:1
D) multiplet with relative area of 1:6:15:20:15:6:1

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
35
Predict the number of signals expected, their splitting, and their relative area in the 1H NMR spectrum of CH3CH2OCH3.

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
36
The protons marked Ha and Hb in the molecule below are ________. 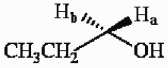
A) chemically equivalent
B) enantiotopic
C) diastereotopic
D) endotopic
E) none of the above
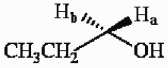
A) chemically equivalent
B) enantiotopic
C) diastereotopic
D) endotopic
E) none of the above

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
37
What compound exhibits only two signals in its 1H NMR spectrum, a triplet and a quintet?
A) BrCH2CH2CH2Br
B) BrCH2CH2CH2Cl
C) (CH3)2CHCH(CH3)2
D) CH3CH2CH2CH3
E) (CH3)2CHOCH(CH3)2
A) BrCH2CH2CH2Br
B) BrCH2CH2CH2Cl
C) (CH3)2CHCH(CH3)2
D) CH3CH2CH2CH3
E) (CH3)2CHOCH(CH3)2

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
38
What is the relationships between Ha and Hb in the following structure? 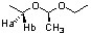
A) homeotopic
B) enantiotopic
C) diastereotopic
D) none of the previous

A) homeotopic
B) enantiotopic
C) diastereotopic
D) none of the previous

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
39
Why is carbon-hydrogen splitting not a major part of 1H NMR spectra?

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
40
What 1H NMR spectral data is expected for the compound shown? 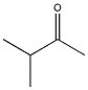
A) 3.8 (1H, septet), 2.1 (3H, s), 1.0 (6H, d)
B) 3.8 (1H, septet), 3.3 (3H, s), 1.0 (6H, d)
C) 3.3 (3H, s), 2.6 (3H, septet), 1.0 (6H, d)
D) 2.6 (1H, septet), 2.1 (3H, s), 1.0 (6H, d)

A) 3.8 (1H, septet), 2.1 (3H, s), 1.0 (6H, d)
B) 3.8 (1H, septet), 3.3 (3H, s), 1.0 (6H, d)
C) 3.3 (3H, s), 2.6 (3H, septet), 1.0 (6H, d)
D) 2.6 (1H, septet), 2.1 (3H, s), 1.0 (6H, d)

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
41
You have a sample and its 1H NMR spectrum. You know your sample contains O atoms but not N atoms, and you suspect that your sample may be an alcohol. What common spectroscopic technique might you use to confirm your suspicion?

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
42
Draw a splitting tree to show the complex splitting pattern for the Hb proton. (Jcis = 10 Hz, trans = 15 Hz and Jgem = 2 Hz 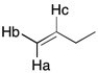


Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
43
How might the proton spectrum of ultrapure dimethylamine, (CH3)2NH, differ from the spectrum of this compound to which D2O has been added?

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
44
Why is carbon-carbon splitting typically not seen in 13C NMR spectra?

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
45
Which compound generates positive peaks for the carbonyl in both its DEPT-90 and DEPT-135 spectra?
A) CH3CH2CHO
B) CH3CH2COCH3
C) CH3CO2CH2CH3
D) CH3CH2CONH2
E) H2CO
A) CH3CH2CHO
B) CH3CH2COCH3
C) CH3CO2CH2CH3
D) CH3CH2CONH2
E) H2CO

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
46
Any process faster than ________ will be recorded as an average by NMR spectroscopy.
A) 0.0005 seconds
B) 0.1 seconds
C) 0.01 seconds
D) 0.001 seconds
E) 1 minute
A) 0.0005 seconds
B) 0.1 seconds
C) 0.01 seconds
D) 0.001 seconds
E) 1 minute

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
47
The chair form of cyclohexane has protons in two distinct environments, axial and equatorial. When the proton NMR of cyclohexane is run on a 100-MHz instrument at 23°C, only one signal for the compound is observed. Explain this apparent contradiction.

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
48
What type of carbon environment does not generate a signal in the DEPT-90 spectrum and gives a negative signal in the DEPT-135 spectrum?
A) quaternary
B) methine
C) methylene
D) methyl
E) carbonyl
A) quaternary
B) methine
C) methylene
D) methyl
E) carbonyl

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
49
How many peaks appear in the proton spin decoupled 13 C NMR spectrum of the compound below? 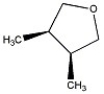
A) 2
B) 3
C) 4
D) 5
E) 6

A) 2
B) 3
C) 4
D) 5
E) 6

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
50
An NMR spectrometer that operates at a frequency of 60 MHz for 13C NMR spectra, operates at what frequency for 1H NMR spectra?
A) 15 MHz
B) 30 MHz
C) 60 MHz
D) 120 MHz
E) 240 MHz
A) 15 MHz
B) 30 MHz
C) 60 MHz
D) 120 MHz
E) 240 MHz

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
51
The 1H NMR spectrum of ethanol is acquired and the hydroxyl signal appears as a singlet instead of a triplet. Offer an explanation.

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
52
What is the approximate chemical shift of an alkynyl carbon in 13C NMR spectroscopy?
A) 10 ppm
B) 30 ppm
C) 70 ppm
D) 120 ppm
E) 200 ppm
A) 10 ppm
B) 30 ppm
C) 70 ppm
D) 120 ppm
E) 200 ppm

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
53
Give one reason why 13C NMR is less sensitive than 1H NMR.

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
54
How many peaks appear in the proton spin decoupled 13 C NMR spectrum of the compound below? 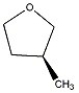
A) 1
B) 2
C) 3
D) 4
E) 5

A) 1
B) 2
C) 3
D) 4
E) 5

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
55
If a signal is observed in the 170 to 200 ppm range in a 13C NMR spectrum, what is the most likely type of functional group associated with that carbon atom?
A) carbonyl carbon
B) allylic carbon
C) aromatic carbon
D) carbon/carbon triple bond
E) carbon/carbon double bond
A) carbonyl carbon
B) allylic carbon
C) aromatic carbon
D) carbon/carbon triple bond
E) carbon/carbon double bond

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
56
Predict the number of signals expected in the proton spin decoupled 13C spectrum of cyclopentane.

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
57
Predict the number of signals expected in the proton spin decoupled 13C NMR spectrum of the compound shown below. 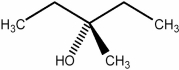


Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
58
What multiplicities are observed in the off-resonance decoupled 13C spectrum of 2,3-dimethyl-but-2-ene?

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
59
What compound exhibits three singlets in its proton spin decoupled 13C NMR spectrum?
A) BrCH2CH2CH2Br
B) BrCH2CH2CH2Cl
C) (CH3)2CHCH(CH3)2
D) CH3CH2CH2CH3
E) (CH3)2CHOCH(CH3)2
A) BrCH2CH2CH2Br
B) BrCH2CH2CH2Cl
C) (CH3)2CHCH(CH3)2
D) CH3CH2CH2CH3
E) (CH3)2CHOCH(CH3)2

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
60
What type of carbon environment does not generate a signal in the DEPT-90 spectrum and gives a positive peak in the DEPT-135 spectrum?
A) quaternary
B) methine
C) methylene
D) methyl
E) carbonyl
A) quaternary
B) methine
C) methylene
D) methyl
E) carbonyl

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
61
Draw the 1H NMR spectrum for the following compound. 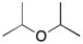


Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
62
How could you tell the difference between these two compounds with 1H NMR? 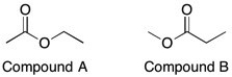


Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
63
Predict the number of signals expected in the proton spin decoupled 13C NMR spectrum of the compound shown below. 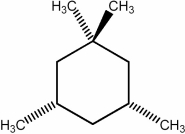


Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
64
Predict the number of distinct quartets expected in the off-resonance decoupled 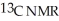
spectrum of the compound shown below.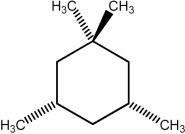
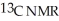
spectrum of the compound shown below.


Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
65
Predict the number of signals expected in the proton spin decoupled 13C NMR spectrum of the compound shown below. 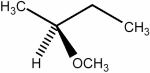


Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
66
Predict the number of signals expected in the proton spin decoupled 13C spectrum of 3-hexanone, CH3CH2COCH2CH2CH3.

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
67
Which carbon signal shows up in the 0-50 ppm region of the proton spin decoupled 13C NMR spectrum? 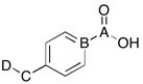
A) A
B) B
C) C
D) D
E) both C and D

A) A
B) B
C) C
D) D
E) both C and D

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
68
How many peaks appear in the proton spin decoupled 13C NMR spectrum of the compound below? 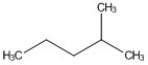


Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
69
How many peaks appear in the proton spin decoupled 13C NMR spectrum of the compound below? 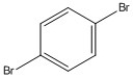


Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
70
How many peaks appear in the proton spin decoupled 13C NMR spectrum of the compound below? 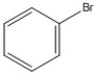


Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
71
Predict the number of distinct quartets expected in the off-resonance decoupled 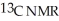
spectrum of the compound shown below.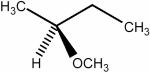
spectrum of the compound shown below.


Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
72
Which carbon signal is furthest down field in a proton spin decoupled 13C NMR spectrum? 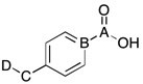
A) A
B) B
C) C
D) D

A) A
B) B
C) C
D) D

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
73
Predict the number of signals expected in the proton spin decoupled 13C NMR spectrum of p-diethylbenzene (1,4-diethylbenzene).

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
74
How many peaks appear in the proton spin decoupled 13C NMR spectrum of the compound below? 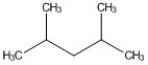


Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
75
Predict the number of signals expected in the proton spin decoupled 13C spectrum of 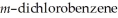
(1,3-dichlorobenzene).
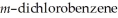
(1,3-dichlorobenzene).

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
76
Predict the number of signals expected in the proton spin decoupled 13C spectrum of 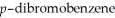
(1,4-dibromobenzene).
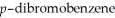
(1,4-dibromobenzene).

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
77
Predict the number of signals expected in the proton spin decoupled 13C NMR spectrum of o-diethylbenzene (1,2-diethylbenzene).

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
78
Draw the 13C NMR spectrum for the following compound. 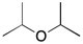


Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
79
How many peaks appear in the proton spin decoupled 13C NMR spectrum of the compound below? 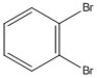


Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
80
Predict the number of distinct quartets expected in the off-resonance decoupled 
spectrum of the compound shown below.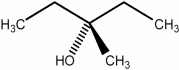
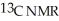
spectrum of the compound shown below.


Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck



