Deck 4: Alkanes and Cycloalkanes
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/66
Play
Full screen (f)
Deck 4: Alkanes and Cycloalkanes
1
What is the structure of 3-ethyl-2,4,6-trimethyloctane?
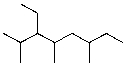
2
What is the structure of 3-ethyl-2-methylpentane?
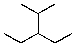
3
What is the structure of [2.2.2]bicyclooctane?

4
What is the parent chain for the following compound? 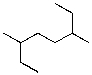
A)octane
B)hexane
C)heptane
D)decane

A)octane
B)hexane
C)heptane
D)decane

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
5
What are the structure and IUPAC names of all of the constitutional isomers of C6H14?

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
6
What is the parent chain for the following compound? 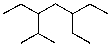


Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
7
What is the IUPAC name for the following compound? 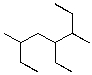
A)2,3,5-triethylhexane
B)2,4,5-triethylhexane
C)2,4-diethyl-5-methylheptane
D)4-ethyl-3,6-dimethyloctane

A)2,3,5-triethylhexane
B)2,4,5-triethylhexane
C)2,4-diethyl-5-methylheptane
D)4-ethyl-3,6-dimethyloctane

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
8
What is the common name of the following substituent? 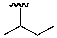
A)isopropyl
B)tert-butyl
C)iso-butyl
D)sec-butyl

A)isopropyl
B)tert-butyl
C)iso-butyl
D)sec-butyl

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
9
What is the IUPAC name for the following compound? 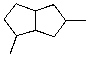


Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
10
What is the IUPAC name for the following compound? 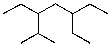
A)3-isopropyl-5-ethylheptane
B)2-methyl-3-ethyl-5-ethylheptane
C)3,5-diethyl-2-methylheptane
D)2-methyl-3,5-diethylheptane

A)3-isopropyl-5-ethylheptane
B)2-methyl-3-ethyl-5-ethylheptane
C)3,5-diethyl-2-methylheptane
D)2-methyl-3,5-diethylheptane

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
11
What is the IUPAC name for the following compound? 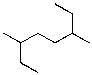
A)2,5-diethylhexane
B)3,6-dimethyloctane
C)2-ethyl-5-methylheptane
D)2,5-dimethylheptane

A)2,5-diethylhexane
B)3,6-dimethyloctane
C)2-ethyl-5-methylheptane
D)2,5-dimethylheptane

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
12
What is the structure of 1-cyclopropyl-3-methylcyclopentane?

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
13
What is the IUPAC name for the following compound? 
A)1-ethyl-2-methylcyclohexane
B)2-ethyl-1-methylcyclohexane
C)1-ethyl-2-methylhexane
D)cyclononane

A)1-ethyl-2-methylcyclohexane
B)2-ethyl-1-methylcyclohexane
C)1-ethyl-2-methylhexane
D)cyclononane

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
14
What is the IUPAC name for the following compound? 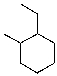


Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
15
What is the relationship between the following two compounds? 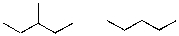
A)Constitutional Isomers
B)The same compound
C)Completely different and not constitutional isomers
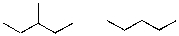
A)Constitutional Isomers
B)The same compound
C)Completely different and not constitutional isomers

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
16
What is the relationship between the following two compounds? 
A)Constitutional Isomers
B)The same compound
C)Completely different and not constitutional isomers

A)Constitutional Isomers
B)The same compound
C)Completely different and not constitutional isomers

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
17
What is the IUPAC name for the following compound? 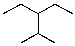


Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
18
What is the parent chain for the following compound? 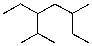


Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
19
What is the relationship between the following two compounds? 
A)Constitutional Isomers
B)The same compound
C)Completely different and not constitutional isomers

A)Constitutional Isomers
B)The same compound
C)Completely different and not constitutional isomers

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
20
What is the structure of an isopropyl group?

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
21
What is the correct stoichiometry of oxygen for the balanced oxidation reaction? 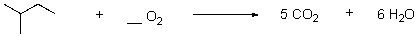
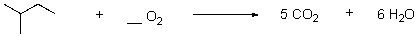

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
22
Draw a Newman projection for the following compound as viewed down the indicated bond. 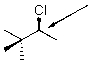


Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
23
Complete the following combustion reaction,assuming that only CO2 and H2O are formed. 


Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
24
What is the relationship between the following two compounds? 
A)Constitutional Isomers
B)The same compound
C)Completely different and not constitutional isomers

A)Constitutional Isomers
B)The same compound
C)Completely different and not constitutional isomers

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
25
Complete the following combustion reaction,assuming that only CO2 and H2O are formed. 


Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
26
Draw the lowest energy conformer of the following compound. 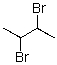


Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
27
Draw a wedge and dash projection of the following Newman projection. 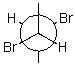


Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
28
Draw the Newman projection of the anti conformer of the following compound. 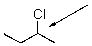


Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
29
What is the correct stoichiometry of oxygen for the balanced oxidation reaction? 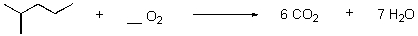
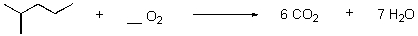

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
30
Draw an energy diagram for propane.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
31
Draw a Newman projection for the following compound as viewed down the indicated bond. 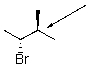


Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
32
Draw a wedge and dash projection of the following Newman projection. 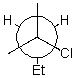


Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
33
What is the relationship between the following two compounds? 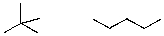
A)Constitutional Isomers
B)The same compound
C)Completely different and not constitutional isomers
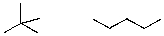
A)Constitutional Isomers
B)The same compound
C)Completely different and not constitutional isomers

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
34
Draw a Newman projection for the following compound as viewed down the indicated bond. 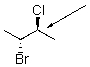


Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
35
What is the energy difference between the staggered and eclipsed conformations of 2,2,-dimethylpropane if the eclipsing cost of Me and H is 6 kJ/mol?

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
36
Draw a wedge and dash projection of the following Newman projection. 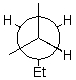


Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
37
Draw the gauche conformer of the following compound. 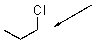
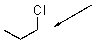

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
38
Draw the lowest energy conformer of the following compound. 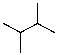


Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
39
Draw the Newman projection for the eclipsed form of propane.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
40
What is the eclipsing cost of Br and H if the energy difference between the staggered and eclipsed conformations of bromoethane is 13 kJ/mol?

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
41
Draw an energy diagram for rotation around the indicated bond of the following compound. 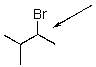


Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
42
Are the two substituents cis or trans? 
A)trans
B)cis
C)neither

A)trans
B)cis
C)neither

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
43
Are the two substituents cis or trans? 
A)trans
B)cis
C)neither

A)trans
B)cis
C)neither

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
44
Draw the most stable chair conformer of the most stable isomer of 1,3,5-trimethylcyclohexane.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
45
Draw the most stable chair conformation of the following cyclohexane. 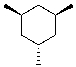


Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
46
Draw both chair conformers for the following compound. 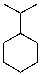


Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
47
Draw the most stable chair conformer of the following compound. 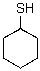


Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
48
Is the following labeled H axial or equatorial? 


Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
49
Draw the chair conformation of cyclohexane.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
50
Draw the most stable chair conformation of the following cyclohexane. 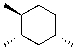


Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
51
Draw a chair conformation of ethylcyclohexane with the ethyl group axial.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
52
Draw both chair conformers for the following compound. 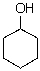


Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
53
Are the two substituents cis or trans? 
A)trans
B)cis
C)neither

A)trans
B)cis
C)neither

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
54
Draw a chair conformation of cyclohexane showing all of the equatorial H's.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
55
Draw the most stable chair conformer of the following compound. 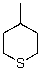


Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
56
Are the two substituents cis or trans? 
A)trans
B)cis
C)neither

A)trans
B)cis
C)neither

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
57
Draw the most stable chair conformer of the most stable isomer of 1-ethyl-3-isopropylcyclohexane.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
58
Draw the lowest energy conformer of the following compound. 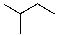


Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
59
Draw a chair conformation of methylcyclohexane.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
60
Draw the other chair conformer of the following compound. 


Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
61
Draw the most stable chair conformer of cis 1,4-diethylcyclohexane.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
62
Which is more stable? 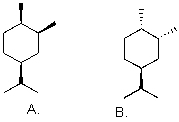
A)A
B)B
C)neither

A)A
B)B
C)neither

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
63
Which is more stable? 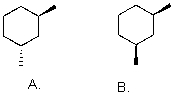
A)A
B)B
C)neither

A)A
B)B
C)neither

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
64
Draw the most stable conformation of trans 1,4-diethylcyclohexane.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
65
Draw the lowest energy chair conformer of the most stable isomer of 4-isopropyl-1,2-dimethylcyclohexane.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
66
Which is more stable? 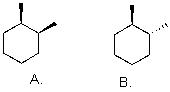
A)A
B)B
C)neither

A)A
B)B
C)neither

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck



