Deck 10: Alkynes
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/161
Play
Full screen (f)
Deck 10: Alkynes
1
Which of the following statements is true about propyne,H-C≡C-CH3?
A)It contains 3 sigma bonds.
B)It contains three pi bonds.
C)The H-C≡C bond angle is about 109.5°.
D)The C≡C-C bond angle is 180°.
E)All carbon-carbon bonds are of equal length.
A)It contains 3 sigma bonds.
B)It contains three pi bonds.
C)The H-C≡C bond angle is about 109.5°.
D)The C≡C-C bond angle is 180°.
E)All carbon-carbon bonds are of equal length.
The C≡C-C bond angle is 180°.
2
What is the hybridization of the carbon atoms numbered 1 and 2 respectively in the following structure? 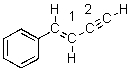
A)sp3,sp2
B)sp2,sp2
C)sp,sp
D)sp2,sp
E)sp,sp2

A)sp3,sp2
B)sp2,sp2
C)sp,sp
D)sp2,sp
E)sp,sp2
sp2,sp
3
For the molecule below,known as 2,4-hexadiyne,which of the following describes the orbital overlap of the C3-C4 sigma bond? 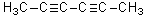
A)sp-sp
B)sp2-sp2
C)sp3-sp3
D)p-p
E)sp3-sp
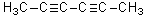
A)sp-sp
B)sp2-sp2
C)sp3-sp3
D)p-p
E)sp3-sp
sp-sp
4
Which of the following is an acceptable structure for 2-hexyne? 
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 161 flashcards in this deck.
Unlock Deck
k this deck
5
What is the correct IUPAC name for the molecule shown below? 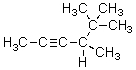
A)2,2,3-Trimethyl-4-hexyne
B)4-tert-Butyl-2-pentyne
C)4,5,5-Trimethyl-2-hexyne
D)4,5,5,5-Tetramethyl-2-pentyne
E)2,2-Dimethyl-3-(1-propynyl)butane

A)2,2,3-Trimethyl-4-hexyne
B)4-tert-Butyl-2-pentyne
C)4,5,5-Trimethyl-2-hexyne
D)4,5,5,5-Tetramethyl-2-pentyne
E)2,2-Dimethyl-3-(1-propynyl)butane

Unlock Deck
Unlock for access to all 161 flashcards in this deck.
Unlock Deck
k this deck
6
What is the correct IUPAC name for the molecule shown below? 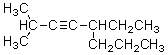
A)2-Methyl-5-propyl-3-heptyne
B)5-Ethyl-2-methyl-3-octyne
C)1-Isopropyl-3-ethyl-1-hexyne
D)6-Methyl-3-propyl-4-heptyne
E)4-Ethyl-7-methyl-5-octyne
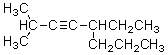
A)2-Methyl-5-propyl-3-heptyne
B)5-Ethyl-2-methyl-3-octyne
C)1-Isopropyl-3-ethyl-1-hexyne
D)6-Methyl-3-propyl-4-heptyne
E)4-Ethyl-7-methyl-5-octyne

Unlock Deck
Unlock for access to all 161 flashcards in this deck.
Unlock Deck
k this deck
7
Provide the systematic IUPAC name for BrCH2CH2C≡CCH2CH3.
A)1-Bromo-3-hexyne
B)6-Bromo-3-hexyne
C)1-Bromo-2-hexyne
D)6-Bromo-4-hexyne
E)1-Bromo-4-hexyne
A)1-Bromo-3-hexyne
B)6-Bromo-3-hexyne
C)1-Bromo-2-hexyne
D)6-Bromo-4-hexyne
E)1-Bromo-4-hexyne

Unlock Deck
Unlock for access to all 161 flashcards in this deck.
Unlock Deck
k this deck
8
What is the correct IUPAC name for the molecule shown below? 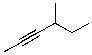
A)4-Ethyl-2-pentyne
B)2-Ethyl-3-pentyne
C)3-Methyl-4-hexyne
D)4-Methyl-2-hexyne
E)sec-Butylpropyne

A)4-Ethyl-2-pentyne
B)2-Ethyl-3-pentyne
C)3-Methyl-4-hexyne
D)4-Methyl-2-hexyne
E)sec-Butylpropyne

Unlock Deck
Unlock for access to all 161 flashcards in this deck.
Unlock Deck
k this deck
9
The C≡C bond of an alkyne is composed of which bond types?
A)three anti-bonds
B)three σ bonds
C)two σ bonds and one π bond
D)one σ bond and two π bonds
E)three π bonds
A)three anti-bonds
B)three σ bonds
C)two σ bonds and one π bond
D)one σ bond and two π bonds
E)three π bonds

Unlock Deck
Unlock for access to all 161 flashcards in this deck.
Unlock Deck
k this deck
10
What is the correct IUPAC name for the molecule shown below? 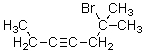
A)6-Bromo-3-octyne
B)6-Bromo-6-methyl-3-heptyne
C)2-Bromo-2-methyl-4-heptyne
D)6-Bromo-6,6-dimethyl-3-hexyne
E)2-Bromo-4-octyne
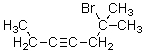
A)6-Bromo-3-octyne
B)6-Bromo-6-methyl-3-heptyne
C)2-Bromo-2-methyl-4-heptyne
D)6-Bromo-6,6-dimethyl-3-hexyne
E)2-Bromo-4-octyne

Unlock Deck
Unlock for access to all 161 flashcards in this deck.
Unlock Deck
k this deck
11
Which of the following is an acceptable structure for 3-sec-butyl-1-heptyne? 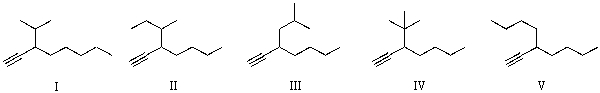
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 161 flashcards in this deck.
Unlock Deck
k this deck
12
Which of following statements best describes the general reactivity of alkynes?
A)An alkyne reacts as a nucleophile,and is therefore electron rich.
B)An alkyne reacts as a nucleophile,and is therefore electron poor.
C)Alkynes fail to undergo electrophilic addition reactions,unlike alkenes.
D)An alkyne reacts as an electrophile,and is therefore electron rich.
E)An alkyne reacts as an electrophile,and is therefore electron poor.
A)An alkyne reacts as a nucleophile,and is therefore electron rich.
B)An alkyne reacts as a nucleophile,and is therefore electron poor.
C)Alkynes fail to undergo electrophilic addition reactions,unlike alkenes.
D)An alkyne reacts as an electrophile,and is therefore electron rich.
E)An alkyne reacts as an electrophile,and is therefore electron poor.

Unlock Deck
Unlock for access to all 161 flashcards in this deck.
Unlock Deck
k this deck
13
What is the correct IUPAC name for the molecule shown below? 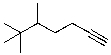
A)5,6,6-Trimethyl-1-heptyne
B)5-tert-Tutyl-1-hexyne
C)2,2,3-Trimethyl-6-heptyne
D)2,2,3-(3-Butynyl)butane
E)sec-Butyl-tert-butylacetylene

A)5,6,6-Trimethyl-1-heptyne
B)5-tert-Tutyl-1-hexyne
C)2,2,3-Trimethyl-6-heptyne
D)2,2,3-(3-Butynyl)butane
E)sec-Butyl-tert-butylacetylene

Unlock Deck
Unlock for access to all 161 flashcards in this deck.
Unlock Deck
k this deck
14
Which of the following is an acceptable structure for hepta-3,6-dien-1-yne? 
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 161 flashcards in this deck.
Unlock Deck
k this deck
15
The sigma bond of an alkyne is formed from the overlap of which orbitals?
A)sp3-sp3
B)p-p
C)sp2-sp2
D)s-s
E)sp-sp
A)sp3-sp3
B)p-p
C)sp2-sp2
D)s-s
E)sp-sp

Unlock Deck
Unlock for access to all 161 flashcards in this deck.
Unlock Deck
k this deck
16
Provide the systematic IUPAC name for Cl3CCH2CH2CH2C≡CH.
A)6,6,6-Trichloro-1-hexyne
B)1,1,1-Trichloro-5-hexyne
C)5,5,5-Trichloro-1-pentyne
D)1-Heptyne
E)Trichlorobutylacetylene
A)6,6,6-Trichloro-1-hexyne
B)1,1,1-Trichloro-5-hexyne
C)5,5,5-Trichloro-1-pentyne
D)1-Heptyne
E)Trichlorobutylacetylene

Unlock Deck
Unlock for access to all 161 flashcards in this deck.
Unlock Deck
k this deck
17
What is the correct IUPAC name for the molecule shown below? 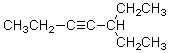
A)1,1-Diethyl-2-pentyne
B)3-(1-Butynyl)pentane
C)5-Ethyl-3-octyne
D)3-Ethyl-4-heptyne
E)5-Ethyl-3-heptyne
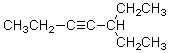
A)1,1-Diethyl-2-pentyne
B)3-(1-Butynyl)pentane
C)5-Ethyl-3-octyne
D)3-Ethyl-4-heptyne
E)5-Ethyl-3-heptyne

Unlock Deck
Unlock for access to all 161 flashcards in this deck.
Unlock Deck
k this deck
18
For the molecule below,known as 2-pentyne,which of the following describes the orbital overlap of the C2-C3 sigma bond? 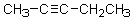
A)sp-sp
B)sp2-sp2
C)sp3-sp3
D)p-p
E)sp3-sp
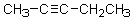
A)sp-sp
B)sp2-sp2
C)sp3-sp3
D)p-p
E)sp3-sp

Unlock Deck
Unlock for access to all 161 flashcards in this deck.
Unlock Deck
k this deck
19
Provide the systematic IUPAC name for HC≡CCH2CH2CH3.
A)Pentyne
B)1-Pentyne
C)Butyne
D)1-Butyne
E)2-Butyne
A)Pentyne
B)1-Pentyne
C)Butyne
D)1-Butyne
E)2-Butyne

Unlock Deck
Unlock for access to all 161 flashcards in this deck.
Unlock Deck
k this deck
20
What is the correct IUPAC name for the molecule shown below? 
A)4,4-Dimethyl-2-pentyne
B)2,2-Dimethyl-4-heptyne
C)1-tertButyl-3-heptyne
D)6,6-Dimethyl-3-heptyne
E)6,6,6-Trimethyl-3-hexyne

A)4,4-Dimethyl-2-pentyne
B)2,2-Dimethyl-4-heptyne
C)1-tertButyl-3-heptyne
D)6,6-Dimethyl-3-heptyne
E)6,6,6-Trimethyl-3-hexyne

Unlock Deck
Unlock for access to all 161 flashcards in this deck.
Unlock Deck
k this deck
21
Sodium amide (NaNH2,sodamide)reacts with terminal alkynes in the role of the
A)Brønsted acid.
B)Brønsted base.
C)reducing agent.
D)catalyst.
E)electrophile.
A)Brønsted acid.
B)Brønsted base.
C)reducing agent.
D)catalyst.
E)electrophile.

Unlock Deck
Unlock for access to all 161 flashcards in this deck.
Unlock Deck
k this deck
22
Which structure shown below represents (Z)-3,5-dichloro-3-hexen-1-yne? 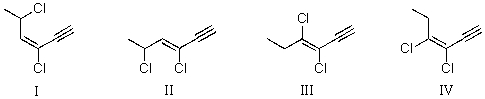
A)I
B)II
C)III
D)IV
E)I and II are both correct

A)I
B)II
C)III
D)IV
E)I and II are both correct

Unlock Deck
Unlock for access to all 161 flashcards in this deck.
Unlock Deck
k this deck
23
Which of the following is an acceptable structure for (2E,4E)-octa-2,4-dien-6-yne? 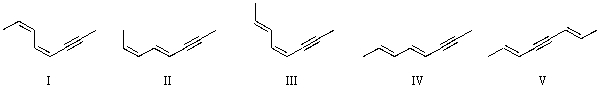
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 161 flashcards in this deck.
Unlock Deck
k this deck
24
What is the correct IUPAC name for the molecule shown below? 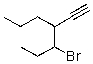
A)3-Bromo-4-acetylenyl-heptane
B)3-(1-Bromopropyl)-1-hexyne
C)3-Bromo-4-propyl-5-hexyne
D)4-Bromo-3-propyl-1-hexyne
E)4-Ethynyl-5-bromo-heptane

A)3-Bromo-4-acetylenyl-heptane
B)3-(1-Bromopropyl)-1-hexyne
C)3-Bromo-4-propyl-5-hexyne
D)4-Bromo-3-propyl-1-hexyne
E)4-Ethynyl-5-bromo-heptane

Unlock Deck
Unlock for access to all 161 flashcards in this deck.
Unlock Deck
k this deck
25
What is the correct IUPAC name for diisobutylacetylene?
A)Diisopropylbutyne
B)2,7-Dimethyl-4-octyne
C)3,6-Dimethyl-4-octyne
D)2,5-Diethyl-3-hexyne
E)2,2,5,5-Tetramethyl-3-hexyne
A)Diisopropylbutyne
B)2,7-Dimethyl-4-octyne
C)3,6-Dimethyl-4-octyne
D)2,5-Diethyl-3-hexyne
E)2,2,5,5-Tetramethyl-3-hexyne

Unlock Deck
Unlock for access to all 161 flashcards in this deck.
Unlock Deck
k this deck
26
What is the correct IUPAC name for the molecule shown below? 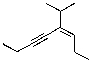
A)(E)-4-isopropyloct-3-en-5-yne
B)(Z)-4-isopropyloct-3-en-5-yne
C)(E)-5-isopropyloct-5-en-3-yne
D)(Z)-5-isopropyloct-5-en-3-yne
E)(E)-4-(2-methylethyl)oct-3-en-5-yne

A)(E)-4-isopropyloct-3-en-5-yne
B)(Z)-4-isopropyloct-3-en-5-yne
C)(E)-5-isopropyloct-5-en-3-yne
D)(Z)-5-isopropyloct-5-en-3-yne
E)(E)-4-(2-methylethyl)oct-3-en-5-yne

Unlock Deck
Unlock for access to all 161 flashcards in this deck.
Unlock Deck
k this deck
27
Rank the following carbanions,with the weakest base first,then by increasing base strength. 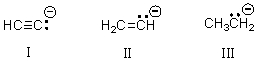
A)I < II < III
B)II < III < I
C)III < II < I
D)III < I < II
E)II < I < III
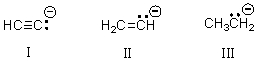
A)I < II < III
B)II < III < I
C)III < II < I
D)III < I < II
E)II < I < III

Unlock Deck
Unlock for access to all 161 flashcards in this deck.
Unlock Deck
k this deck
28
Which of the circled hydrogen atoms is the most acidic? 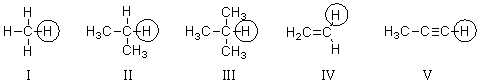
A)I
B)II
C)III
D)IV
E)V
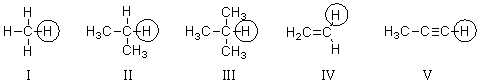
A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 161 flashcards in this deck.
Unlock Deck
k this deck
29
Which of the bases below would quantitatively deprotonate a terminal alkyne?
A)BuLi
B)NH3
C)NaOH
D)NaOCH2CH3
E)t-BuOK
A)BuLi
B)NH3
C)NaOH
D)NaOCH2CH3
E)t-BuOK

Unlock Deck
Unlock for access to all 161 flashcards in this deck.
Unlock Deck
k this deck
30
What is the correct IUPAC name for the molecule shown below? 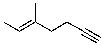
A)(E)-5-methyl-5-hepten-1-yne
B)(Z)-5-methyl-5-hepten-1-yne
C)(E)-3-methyl-2-hepten-6-yne
D)(Z)-3-methyl-2-hepten-6-yne
E)(E)-2-butynyl-2-butene

A)(E)-5-methyl-5-hepten-1-yne
B)(Z)-5-methyl-5-hepten-1-yne
C)(E)-3-methyl-2-hepten-6-yne
D)(Z)-3-methyl-2-hepten-6-yne
E)(E)-2-butynyl-2-butene

Unlock Deck
Unlock for access to all 161 flashcards in this deck.
Unlock Deck
k this deck
31
Which of the following is an acceptable structure for (R)-5-bromohept-2-yne. 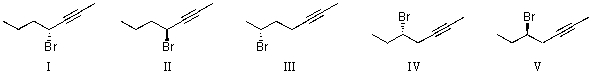
A)I
B)II
C)III
D)IV
E)V
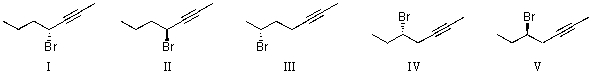
A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 161 flashcards in this deck.
Unlock Deck
k this deck
32
What is the correct IUPAC name for the molecule shown below? 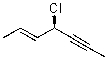
A)(R,Z)-4-chlorohept-5-en-2-yne
B)(S,E)-4-chlorohept-5-en-2-yne
C)(S,E)-4-chlorohept-2-en-5-yne
D)(R,E)-4-chlorohept-5-en-2-yne
E)(R,E)-4-chlorohept-2-en-5-yne

A)(R,Z)-4-chlorohept-5-en-2-yne
B)(S,E)-4-chlorohept-5-en-2-yne
C)(S,E)-4-chlorohept-2-en-5-yne
D)(R,E)-4-chlorohept-5-en-2-yne
E)(R,E)-4-chlorohept-2-en-5-yne

Unlock Deck
Unlock for access to all 161 flashcards in this deck.
Unlock Deck
k this deck
33
Provide the systematic IUPAC name for the molecule below: CH3C(CH3)2CH2C≡CCH2CH(CH2CH3)CH3
A)2,7,7-Trimethyl-5-nonyne
B)2-Ethyl-7,7-dimethyl-4-octyne
C)2,2,7-Trimethyl-4-nonyne
D)7-Ethyl-2,2-trimethyl-4-octyne
E)6-Undecyne
A)2,7,7-Trimethyl-5-nonyne
B)2-Ethyl-7,7-dimethyl-4-octyne
C)2,2,7-Trimethyl-4-nonyne
D)7-Ethyl-2,2-trimethyl-4-octyne
E)6-Undecyne

Unlock Deck
Unlock for access to all 161 flashcards in this deck.
Unlock Deck
k this deck
34
Which of the following is an acceptable structure for octa-3,6-dien-1-yne?
A)HC≡CCH=CHCH=CHCH2CH3
B)CH3CH=CHCH2C≡CC≡CH
C)CH3CH=CHCH2CH=CHC≡CH
D)CH3C≡CCH=CHCH=CHCH3
E)H2C=CHC≡CCH2CH=CHCH3
A)HC≡CCH=CHCH=CHCH2CH3
B)CH3CH=CHCH2C≡CC≡CH
C)CH3CH=CHCH2CH=CHC≡CH
D)CH3C≡CCH=CHCH=CHCH3
E)H2C=CHC≡CCH2CH=CHCH3

Unlock Deck
Unlock for access to all 161 flashcards in this deck.
Unlock Deck
k this deck
35
Provide the systematic IUPAC name for Cl3C(CH2)4C≡CH.
A)4,4,4-Trichloro-1-butyne
B)1,1,1-Trichloro-6-heptyne
C)1,1,1-Trichloro-5-heptyne
D)6,6,6-Trichloro-1-hexyne
E)7,7,7-Trichloro-1-heptyne
A)4,4,4-Trichloro-1-butyne
B)1,1,1-Trichloro-6-heptyne
C)1,1,1-Trichloro-5-heptyne
D)6,6,6-Trichloro-1-hexyne
E)7,7,7-Trichloro-1-heptyne

Unlock Deck
Unlock for access to all 161 flashcards in this deck.
Unlock Deck
k this deck
36
What is the correct systematic IUPAC name for di-sec-butylacetylene?
A)2-Ethyl-5-methyl-3-heptyne
B)2,7-Dimethyl-4-octyne
C)3,6-Dimethyl-4-octyne
D)2,5-Diethyl-3-hexyne
E)2,2,5,5-Tetramethyl-3-hexyne
A)2-Ethyl-5-methyl-3-heptyne
B)2,7-Dimethyl-4-octyne
C)3,6-Dimethyl-4-octyne
D)2,5-Diethyl-3-hexyne
E)2,2,5,5-Tetramethyl-3-hexyne

Unlock Deck
Unlock for access to all 161 flashcards in this deck.
Unlock Deck
k this deck
37
Rank the following acids,with the strongest acid first,then by decreasing acidity. 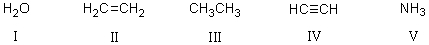
A)V > I > IV > II > III
B)III > IV > II > I > V
C)V > I > III > II > IV
D)I > IV > V > II > III
E)IV > I > V > II > III
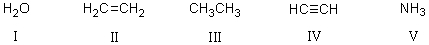
A)V > I > IV > II > III
B)III > IV > II > I > V
C)V > I > III > II > IV
D)I > IV > V > II > III
E)IV > I > V > II > III

Unlock Deck
Unlock for access to all 161 flashcards in this deck.
Unlock Deck
k this deck
38
Provide the systematic IUPAC name for (CH3)2CHC≡CCH2C(CH3)3.
A)1,1,5,5,5-Pentamethyl-2-pentyne
B)1,1,1,5,5-Pentamethyl-3-pentyne
C)2,2,6-trimethyl-4-heptyne
D)2,6,6-trimethyl-3-heptyne
E)tert-butylisopropylacetylene
A)1,1,5,5,5-Pentamethyl-2-pentyne
B)1,1,1,5,5-Pentamethyl-3-pentyne
C)2,2,6-trimethyl-4-heptyne
D)2,6,6-trimethyl-3-heptyne
E)tert-butylisopropylacetylene

Unlock Deck
Unlock for access to all 161 flashcards in this deck.
Unlock Deck
k this deck
39
Which of the following is an acceptable structure for 2,5,5?trimethylhept?3?yne
A)(CH3CH2)CH(CH3)C≡CCH2CH(CH3)2
B)(CH3CH2)C(CH3)2C≡CCH(CH3)2
C)(CH3CH2)2C(CH3)C≡CCH2CH3
D)CH3CH2C(CH3)2C≡CC(CH3)3
E)(CH3CH2CH2)CH(CH3)C≡CC(CH3)3
A)(CH3CH2)CH(CH3)C≡CCH2CH(CH3)2
B)(CH3CH2)C(CH3)2C≡CCH(CH3)2
C)(CH3CH2)2C(CH3)C≡CCH2CH3
D)CH3CH2C(CH3)2C≡CC(CH3)3
E)(CH3CH2CH2)CH(CH3)C≡CC(CH3)3

Unlock Deck
Unlock for access to all 161 flashcards in this deck.
Unlock Deck
k this deck
40
Rank the following bases,with the strongest base first,then by decreasing basicity. 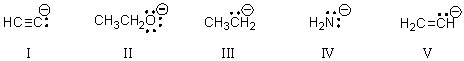
A)III > I > V > II > IV
B)III > V > IV > I> II
C)V > I > III > II > IV
D)III > IV > II > V > I
E)IV > II > I > III > V
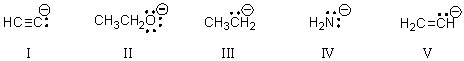
A)III > I > V > II > IV
B)III > V > IV > I> II
C)V > I > III > II > IV
D)III > IV > II > V > I
E)IV > II > I > III > V

Unlock Deck
Unlock for access to all 161 flashcards in this deck.
Unlock Deck
k this deck
41
Select the reagent(s)expected to accomplish the transformation shown below. 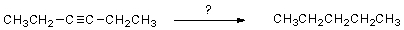
A)H2,Ni
B)H2,Ni2B
C)Na,NH3(l)
D)A and B
E)B and C
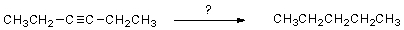
A)H2,Ni
B)H2,Ni2B
C)Na,NH3(l)
D)A and B
E)B and C

Unlock Deck
Unlock for access to all 161 flashcards in this deck.
Unlock Deck
k this deck
42
Select the best explanation for why methanol,CH3OH,cannot be used as a solvent for the deprotonation of a terminal alkyne by sodium amide,NaNH2.
A)Sodium amide is not a strong enough base to deprotonate the alkyne.
B)Sodium amide in methanol reduces alkynes to alkenes.
C)Methanol is a poor solvent for dissolving alkynes.
D)Methanol is more acidic than the alkyne,and will be deprotonated instead.
E)Methanol is toxic,and should be avoided when possible.
A)Sodium amide is not a strong enough base to deprotonate the alkyne.
B)Sodium amide in methanol reduces alkynes to alkenes.
C)Methanol is a poor solvent for dissolving alkynes.
D)Methanol is more acidic than the alkyne,and will be deprotonated instead.
E)Methanol is toxic,and should be avoided when possible.

Unlock Deck
Unlock for access to all 161 flashcards in this deck.
Unlock Deck
k this deck
43
When preparing terminal alkynes by an elimination reaction,sodium amide (NaNH2)dissolved in liquid ammonia (NH3)is used most frequently.Which of the following statements offers the best explanation for the above statement?
A)The above statement is false;terminal alkynes are not produced under the given conditions.
B)Sodium amide deprotonates the terminal alkyne,driving formation of the alkynide ion.
C)Only sodium amide is a strong enough base to deprotonate a carbon.
D)Terminal alkynes are more stable than the internal alkynes,and are always the favored product.
E)Steric hindrance favors preparation of the less substituted terminal alkyne.
A)The above statement is false;terminal alkynes are not produced under the given conditions.
B)Sodium amide deprotonates the terminal alkyne,driving formation of the alkynide ion.
C)Only sodium amide is a strong enough base to deprotonate a carbon.
D)Terminal alkynes are more stable than the internal alkynes,and are always the favored product.
E)Steric hindrance favors preparation of the less substituted terminal alkyne.

Unlock Deck
Unlock for access to all 161 flashcards in this deck.
Unlock Deck
k this deck
44
For the reaction sequence below,select the expected major product. 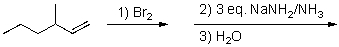
A)3-methylhexane
B)1-bromo-3-methylhexene
C)2-bromo-3-methylhexene
D)3-methyl-1-hexyne
E)3-methyl-2-hexyne
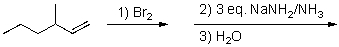
A)3-methylhexane
B)1-bromo-3-methylhexene
C)2-bromo-3-methylhexene
D)3-methyl-1-hexyne
E)3-methyl-2-hexyne

Unlock Deck
Unlock for access to all 161 flashcards in this deck.
Unlock Deck
k this deck
45
Of the species listed below,select those less basic than acetylide?
A)BuLi
B)NaNH2
C)NaOCH3
D)both A and C
E)both B and C
A)BuLi
B)NaNH2
C)NaOCH3
D)both A and C
E)both B and C

Unlock Deck
Unlock for access to all 161 flashcards in this deck.
Unlock Deck
k this deck
46
Which statement below best explains acetylene's Ka being greater than ethylene's?
A)Acetylide anions are resonance stabilized.
B)The 4 electrons of the acetylide anion better stabilize a negative charge.
C)The electronegativity of sp carbons being greater than sp2 carbons.
D)The electronegativity of sp carbons being less than sp2 carbons.
E)Acetylene has only two hydrogen atoms while ethylene has four.
A)Acetylide anions are resonance stabilized.
B)The 4 electrons of the acetylide anion better stabilize a negative charge.
C)The electronegativity of sp carbons being greater than sp2 carbons.
D)The electronegativity of sp carbons being less than sp2 carbons.
E)Acetylene has only two hydrogen atoms while ethylene has four.

Unlock Deck
Unlock for access to all 161 flashcards in this deck.
Unlock Deck
k this deck
47
Reduction of 1 mole of (3E,5Z)-3-methylhepta-3,5-dien-1-yne will require how many moles of hydrogen (H2)?
A)1
B)2
C)3
D)4
E)5
A)1
B)2
C)3
D)4
E)5

Unlock Deck
Unlock for access to all 161 flashcards in this deck.
Unlock Deck
k this deck
48
Which of the bases below would result in the greatest deprotonation of the alkyne,shown in the reaction below? 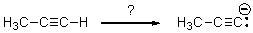
A)NaOCH2CH3 (sodium ethoxide)
B)t-BuONa (sodium tert-butoxide)
C)NaH (sodium hydride)
D)NaHCO3 (sodium bicarbonate)
E)NaOH (sodium hydroxide)
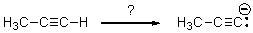
A)NaOCH2CH3 (sodium ethoxide)
B)t-BuONa (sodium tert-butoxide)
C)NaH (sodium hydride)
D)NaHCO3 (sodium bicarbonate)
E)NaOH (sodium hydroxide)

Unlock Deck
Unlock for access to all 161 flashcards in this deck.
Unlock Deck
k this deck
49
Select the best reagent expected to convert 3-heptyne to cis-3-heptene.
A)NaNH2,NH3
B)Na,NH3
C)H2,Lindlar's catalyst
D)Both A and C
E)Both B and C
A)NaNH2,NH3
B)Na,NH3
C)H2,Lindlar's catalyst
D)Both A and C
E)Both B and C

Unlock Deck
Unlock for access to all 161 flashcards in this deck.
Unlock Deck
k this deck
50
Which of the reagents below would convert 2-pentyne to trans-2-pentene?
A)NaNH2,NH3
B)Na,NH3
C)H2,Lindlar's catalyst
D)H2,Pd/C
E)H2O,HgSO4/H2SO4
A)NaNH2,NH3
B)Na,NH3
C)H2,Lindlar's catalyst
D)H2,Pd/C
E)H2O,HgSO4/H2SO4

Unlock Deck
Unlock for access to all 161 flashcards in this deck.
Unlock Deck
k this deck
51
The major result of treating 1-butyne with 6M aqueous NaOH would be:
A)the production of the sodium acetylide.
B)the production of an alkene.
C)the production of an alkane.
D)the production of an enol.
E)nothing,as the alkyne would not react.
A)the production of the sodium acetylide.
B)the production of an alkene.
C)the production of an alkane.
D)the production of an enol.
E)nothing,as the alkyne would not react.

Unlock Deck
Unlock for access to all 161 flashcards in this deck.
Unlock Deck
k this deck
52
Which of the circled hydrogen atoms is the least acidic? 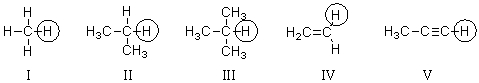
A)I
B)II
C)III
D)IV
E)V
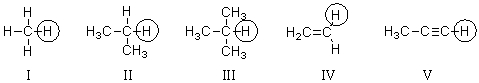
A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 161 flashcards in this deck.
Unlock Deck
k this deck
53
Treatment of 2,2-dibromobutane with molten KOH at 200°C would be expected to produce which of the following:
A)(E)-2-bromo-2-butene
B)(Z)-2-bromo-2-butyne
C)1,2-butadiene
D)1-butyne
E)2-butyne
A)(E)-2-bromo-2-butene
B)(Z)-2-bromo-2-butyne
C)1,2-butadiene
D)1-butyne
E)2-butyne

Unlock Deck
Unlock for access to all 161 flashcards in this deck.
Unlock Deck
k this deck
54
For the reaction shown,select the expected major organic product. 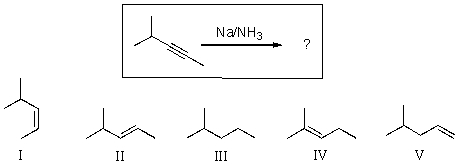
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 161 flashcards in this deck.
Unlock Deck
k this deck
55
For the reaction shown,select the expected major organic product. 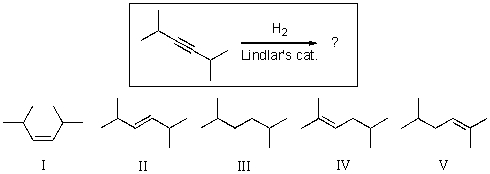
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 161 flashcards in this deck.
Unlock Deck
k this deck
56
For the reaction shown,select the expected major organic product. 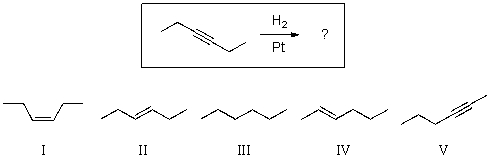
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 161 flashcards in this deck.
Unlock Deck
k this deck
57
For the transformation shown below,select the expected major product. 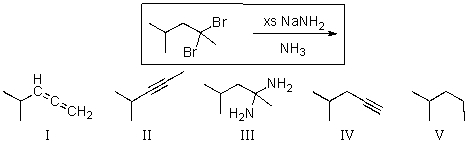
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 161 flashcards in this deck.
Unlock Deck
k this deck
58
Select the best reagent to convert 3-heptyne to trans-3-heptene?
A)Na/NH3
B)1 eq.NaNH2,NH3
C)xs NaNH2,NH3
D)H2/Pt
E)H2/Lindlar's catalyst
A)Na/NH3
B)1 eq.NaNH2,NH3
C)xs NaNH2,NH3
D)H2/Pt
E)H2/Lindlar's catalyst

Unlock Deck
Unlock for access to all 161 flashcards in this deck.
Unlock Deck
k this deck
59
What are the products of the reaction shown below? CH3OH + CH3C≡C-Na+ → ?
A)CH3C≡CCH3 + Na+OH-
B)CH3C≡CH + CH3O-Na+
C)CH3C≡COCH3 + Na+OH-
D)CH3OC≡CH + Na+CH3-
E)no reaction
A)CH3C≡CCH3 + Na+OH-
B)CH3C≡CH + CH3O-Na+
C)CH3C≡COCH3 + Na+OH-
D)CH3OC≡CH + Na+CH3-
E)no reaction

Unlock Deck
Unlock for access to all 161 flashcards in this deck.
Unlock Deck
k this deck
60
For the reaction shown below,select the expected major product. 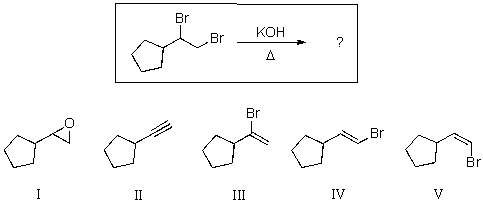
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 161 flashcards in this deck.
Unlock Deck
k this deck
61
For the reaction shown below,what is the IUPAC name of the expected product? 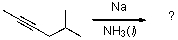
A)2-methylhexane
B)(Z)-2-methyl-4-hexene
C)(E)-2-methyl-4-hexene
D)(Z)-5-methyl-2-hexene
E)(E)-5-methyl-2-hexene
A)2-methylhexane
B)(Z)-2-methyl-4-hexene
C)(E)-2-methyl-4-hexene
D)(Z)-5-methyl-2-hexene
E)(E)-5-methyl-2-hexene

Unlock Deck
Unlock for access to all 161 flashcards in this deck.
Unlock Deck
k this deck
62
The expected major product from the treatment of 1-pentyne with 2 equivalents of HBr is:
A)1-bromo-1-pentene
B)2-bromo-1-pentene
C)1,1-dibromopentane
D)2,2-dibromopentane
E)1,2-dibromopentane
A)1-bromo-1-pentene
B)2-bromo-1-pentene
C)1,1-dibromopentane
D)2,2-dibromopentane
E)1,2-dibromopentane

Unlock Deck
Unlock for access to all 161 flashcards in this deck.
Unlock Deck
k this deck
63
For the reaction below,select the structure of the expected major organic product. 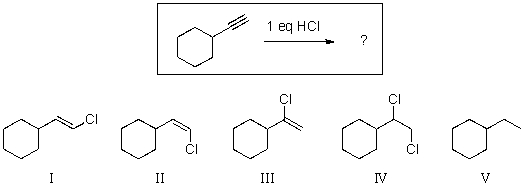
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 161 flashcards in this deck.
Unlock Deck
k this deck
64
Reaction of (3E,5Z)-3-methylhepta-3,5-dien-1-yne with H2 and Pd/C will produce which of the compounds below?
A)1-sec-Butylbutane
B)2-Butylbutane2
C)3-Methylheptane
D)3-Methyl-1-heptyne
E)(3E,5Z)-3-Bethyl-1,3,5-heptatriene
A)1-sec-Butylbutane
B)2-Butylbutane2
C)3-Methylheptane
D)3-Methyl-1-heptyne
E)(3E,5Z)-3-Bethyl-1,3,5-heptatriene

Unlock Deck
Unlock for access to all 161 flashcards in this deck.
Unlock Deck
k this deck
65
What is the expected major organic product from treatment of 4-methyl-2-pentyne with excess hydrogen in the presence of a platinum catalyst?
A)(E)-4-methyl-2-pentene
B)(Z)-4-methyl-2-pentene
C)2-methylpentane
D)4-methylpentane
E)Equal mixture of A and B
A)(E)-4-methyl-2-pentene
B)(Z)-4-methyl-2-pentene
C)2-methylpentane
D)4-methylpentane
E)Equal mixture of A and B

Unlock Deck
Unlock for access to all 161 flashcards in this deck.
Unlock Deck
k this deck
66
Complete hydrogenation of a mixture of 1-octyne,2-octyne,and 3-octyne,in the presence of a palladium catalyst,would produce how many distinct eight-carbon hydrocarbon products?
A)1
B)2
C)3
D)6
E)8
A)1
B)2
C)3
D)6
E)8

Unlock Deck
Unlock for access to all 161 flashcards in this deck.
Unlock Deck
k this deck
67
The expected major product from the treatment of 1-pentyne with 1 equivalent of HBr is:
A)1-bromo-1-pentene
B)2-bromo-1-pentene
C)1,1-dibromopentane
D)2,2-dibromopentane
E)1,2-dibromopentane
A)1-bromo-1-pentene
B)2-bromo-1-pentene
C)1,1-dibromopentane
D)2,2-dibromopentane
E)1,2-dibromopentane

Unlock Deck
Unlock for access to all 161 flashcards in this deck.
Unlock Deck
k this deck
68
Select the reagent(s)expected to accomplish the transformation shown below. 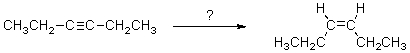
A)H2,Ni
B)H2,Ni2B
C)H2,Lindlar's catalyst
D)A and B
E)B and C
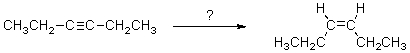
A)H2,Ni
B)H2,Ni2B
C)H2,Lindlar's catalyst
D)A and B
E)B and C

Unlock Deck
Unlock for access to all 161 flashcards in this deck.
Unlock Deck
k this deck
69
Which of the compounds shown below would be the most likely product expected from the reaction scheme shown? 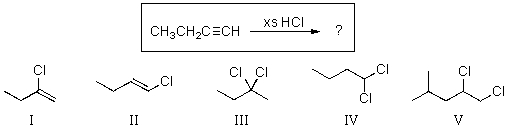
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 161 flashcards in this deck.
Unlock Deck
k this deck
70
Of the reaction conditions provided below,which would be expected to convert 1 mole of 4-methyl-1-pentyne into 2-methylpentane?
A)H2,Lindlar's catalyst
B)Na,NH3(l)
C)2 moles of HCl
D)2 moles H2,Pt
E)1 mole H2,Pt
A)H2,Lindlar's catalyst
B)Na,NH3(l)
C)2 moles of HCl
D)2 moles H2,Pt
E)1 mole H2,Pt

Unlock Deck
Unlock for access to all 161 flashcards in this deck.
Unlock Deck
k this deck
71
For the reaction shown below,the resulting stereochemistry of the expected product is best described as: 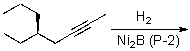
A)(R,E)
B)(S,E)
C)(R,Z)
D)(S,Z)
E)only (S)
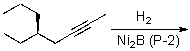
A)(R,E)
B)(S,E)
C)(R,Z)
D)(S,Z)
E)only (S)

Unlock Deck
Unlock for access to all 161 flashcards in this deck.
Unlock Deck
k this deck
72
Reaction of 3,4,5-trimethyl-4-hexen-1-yne with H2 and Pd/C will produce which of the following compounds?
A)2,3,4-Trimethylhexane
B)3,4,5-Trimethylhexane
C)2,3,4-Trimethyl-1-hexene
D)3,4,5-Trimethyl-1-hexyne
E)2,3,4-Trimethyl-5-hexyne2
A)2,3,4-Trimethylhexane
B)3,4,5-Trimethylhexane
C)2,3,4-Trimethyl-1-hexene
D)3,4,5-Trimethyl-1-hexyne
E)2,3,4-Trimethyl-5-hexyne2

Unlock Deck
Unlock for access to all 161 flashcards in this deck.
Unlock Deck
k this deck
73
What is the expected major organic product from treatment of 4-methyl-2-pentyne with sodium metal in liquid ammonia?
A)(E)-4-methyl-2-pentene
B)(Z)-4-methyl-2-pentene
C)(E)-2-methyl-2-pentene
D)(Z)-2-methyl-2-pentene
E)2-methylpentane
A)(E)-4-methyl-2-pentene
B)(Z)-4-methyl-2-pentene
C)(E)-2-methyl-2-pentene
D)(Z)-2-methyl-2-pentene
E)2-methylpentane

Unlock Deck
Unlock for access to all 161 flashcards in this deck.
Unlock Deck
k this deck
74
What is the expected major organic product from treatment of 4-methyl-2-pentyne with hydrogen in the presence of Lindlar's catalyst?
A)(E)-4-methyl-2-pentene
B)(Z)-4-methyl-2-pentene
C)(E)-2-methyl-2-pentene
D)(Z)-2-methyl-2-pentene
E)2-methylpentane
A)(E)-4-methyl-2-pentene
B)(Z)-4-methyl-2-pentene
C)(E)-2-methyl-2-pentene
D)(Z)-2-methyl-2-pentene
E)2-methylpentane

Unlock Deck
Unlock for access to all 161 flashcards in this deck.
Unlock Deck
k this deck
75
Which reagents shown below would be expected to convert 2-pentyne to (Z)-2-pentene?
A)H2,Pt
B)Na,NH3
C)H2,Lindlar's catalyst
D)Xs HCl
E)HgSO4,H2O
A)H2,Pt
B)Na,NH3
C)H2,Lindlar's catalyst
D)Xs HCl
E)HgSO4,H2O

Unlock Deck
Unlock for access to all 161 flashcards in this deck.
Unlock Deck
k this deck
76
The expected major product from treatment of 1-pentyne with excess HBr in the presence of peroxides is:
A)1-bromo-1-pentene
B)2-bromo-1-pentene
C)1,1-dibromopentane
D)2,2-dibromopentane
E)1,2-dibromopentane
A)1-bromo-1-pentene
B)2-bromo-1-pentene
C)1,1-dibromopentane
D)2,2-dibromopentane
E)1,2-dibromopentane

Unlock Deck
Unlock for access to all 161 flashcards in this deck.
Unlock Deck
k this deck
77
In a dissolving metal reduction of an alkyne,a postulated intermediate is the trans alkenyl radical,shown below.In which orbital type would the unpaired electron be located? 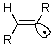
A)sp
B)sp2
C)sp3
D)p
E)s

A)sp
B)sp2
C)sp3
D)p
E)s

Unlock Deck
Unlock for access to all 161 flashcards in this deck.
Unlock Deck
k this deck
78
In the reaction between an alkyne and Na metal in liquid ammonia,Na's role is as the:
A)Brønsted acid.
B)Brønsted base.
C)reducing agent.
D)catalyst.
E)electrophile.
A)Brønsted acid.
B)Brønsted base.
C)reducing agent.
D)catalyst.
E)electrophile.

Unlock Deck
Unlock for access to all 161 flashcards in this deck.
Unlock Deck
k this deck
79
For the reaction shown below,the resulting stereochemistry of the expected product is best described as: 
A)only (S)
B)only (R)
C)racemic
D)meso
E)achiral

A)only (S)
B)only (R)
C)racemic
D)meso
E)achiral

Unlock Deck
Unlock for access to all 161 flashcards in this deck.
Unlock Deck
k this deck
80
Select the reagent(s)expected to accomplish the transformation shown below. 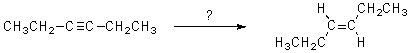
A)H2,Pd
B)H2,Lindlar's catalyst
C)Na,NH3(l)
D)A and B
E)B and C
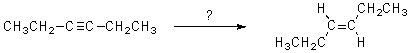
A)H2,Pd
B)H2,Lindlar's catalyst
C)Na,NH3(l)
D)A and B
E)B and C

Unlock Deck
Unlock for access to all 161 flashcards in this deck.
Unlock Deck
k this deck



