Deck 26: Amino Acids,peptides,proteins,and Nucleic Acids: Nitrogen-Containing Polymers in Nature
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/39
Play
Full screen (f)
Deck 26: Amino Acids,peptides,proteins,and Nucleic Acids: Nitrogen-Containing Polymers in Nature
1
Which of the following is least likely to be found in nature?
A)
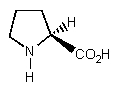
B)
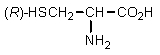
C)
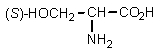
D)
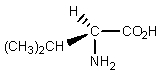
E)
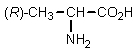
A)

B)
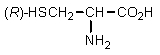
C)
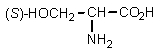
D)

E)
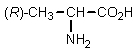
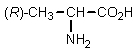
2
Which of the following amino acids would have the highest isoelectric point?
A) Lysine
B) Glutamine
C) Aspartic acid
D) Alanine
E) Tryptophan
A) Lysine
B) Glutamine
C) Aspartic acid
D) Alanine
E) Tryptophan
Lysine
3
Why is the following not a good two step route for the preparation of pure L-alanine? 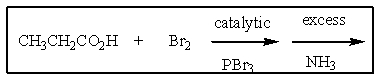
A) The bromination is not selective (the CH3 and CH2 groups are both brominated).
B) Ammonia is not sufficiently nucleophillic to react with a secondary bromide.
C) Only the CH3 group is brominated.
D) The product will be racemic.
E) Propanamide would be the major product.

A) The bromination is not selective (the CH3 and CH2 groups are both brominated).
B) Ammonia is not sufficiently nucleophillic to react with a secondary bromide.
C) Only the CH3 group is brominated.
D) The product will be racemic.
E) Propanamide would be the major product.
The product will be racemic.
4
Disulfide bonds in proteins
A) result from oxidation of thiols (RSH).
B) function to help maintain the shape of proteins.
C) can be broken by reduction reactions.
D) link two cysteine amino acid residues.
E) All of these are true.
A) result from oxidation of thiols (RSH).
B) function to help maintain the shape of proteins.
C) can be broken by reduction reactions.
D) link two cysteine amino acid residues.
E) All of these are true.

Unlock Deck
Unlock for access to all 39 flashcards in this deck.
Unlock Deck
k this deck
5
Why is the additional (ring)nitrogen in tryptophan not very basic? 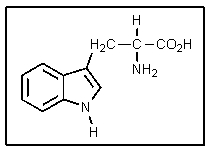
A) Cyclic amines are not basic.
B) It is too far from the carboxyl group to be effected.
C) Aromatic amines are weakly basic because of resonance.
D) The additional amine group is offset by an additional carboxylic acid group.
E) It is sp2 hybridized as therefore not as basic.

A) Cyclic amines are not basic.
B) It is too far from the carboxyl group to be effected.
C) Aromatic amines are weakly basic because of resonance.
D) The additional amine group is offset by an additional carboxylic acid group.
E) It is sp2 hybridized as therefore not as basic.

Unlock Deck
Unlock for access to all 39 flashcards in this deck.
Unlock Deck
k this deck
6
The primary structure of a protein refers to the
A) sequence of amino acids.
B) overall 3-dimensional shape.
C) localized shape of the backbone.
D) degree of aggregation with other proteins.
E) structure of the active site.
A) sequence of amino acids.
B) overall 3-dimensional shape.
C) localized shape of the backbone.
D) degree of aggregation with other proteins.
E) structure of the active site.

Unlock Deck
Unlock for access to all 39 flashcards in this deck.
Unlock Deck
k this deck
7
What material can be used repetitively (on a given sample)to sequence a polypeptide from the N-terminal end?
A) Chymotrypsin
B) Sanger reagent
C) Trypsin
D) Edman reagent
E) Carboxypeptidase
A) Chymotrypsin
B) Sanger reagent
C) Trypsin
D) Edman reagent
E) Carboxypeptidase

Unlock Deck
Unlock for access to all 39 flashcards in this deck.
Unlock Deck
k this deck
8
Nearly all amino acids immediately release N2 gas upon diazotization (treatment with aqueous HCl and sodium nitrite).Which amino acid would not?
A) Lysine
B) Histidine
C) Tyrosine
D) Cysteine
E) Proline
A) Lysine
B) Histidine
C) Tyrosine
D) Cysteine
E) Proline

Unlock Deck
Unlock for access to all 39 flashcards in this deck.
Unlock Deck
k this deck
9
Which of the following amino acids is theoretically capable of existing in two diastereomeric forms?
A) Cysteine
B) Threonine
C) Leucine
D) Serine
E) Tryptophan
A) Cysteine
B) Threonine
C) Leucine
D) Serine
E) Tryptophan

Unlock Deck
Unlock for access to all 39 flashcards in this deck.
Unlock Deck
k this deck
10
What is dicyclohexylcarbodiimide (DCC)used for in peptide synthesis?
A) DCC protects the amino group of the intended N-terminal amino acid.
B) DCC activates the carboxyl group toward nucleophillic attack.
C) DCC cleaves the blocking groups from the final peptide.
D) DCC is the resin (solid support)used to anchor the growing polypeptide.
E) DCC removes the peptide from the resin at the conclusion of the synthesis.
A) DCC protects the amino group of the intended N-terminal amino acid.
B) DCC activates the carboxyl group toward nucleophillic attack.
C) DCC cleaves the blocking groups from the final peptide.
D) DCC is the resin (solid support)used to anchor the growing polypeptide.
E) DCC removes the peptide from the resin at the conclusion of the synthesis.

Unlock Deck
Unlock for access to all 39 flashcards in this deck.
Unlock Deck
k this deck
11
What is the name of the DNA base shown? 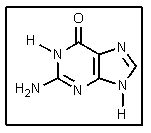
A) Guanine
B) Cytosine
C) Adenine
D) Uracil
E) Thymine

A) Guanine
B) Cytosine
C) Adenine
D) Uracil
E) Thymine

Unlock Deck
Unlock for access to all 39 flashcards in this deck.
Unlock Deck
k this deck
12
What would be the abbreviated name of the following polypeptide? 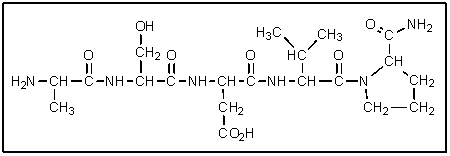
A) val-thr-glu-ala-gln
B) ala-thr-glu-val-gln
C) met-ser-asp-leu-pro-NH2
D) ala-ser-asp-val-gln
E) ala-ser-asp-val-pro-NH2

A) val-thr-glu-ala-gln
B) ala-thr-glu-val-gln
C) met-ser-asp-leu-pro-NH2
D) ala-ser-asp-val-gln
E) ala-ser-asp-val-pro-NH2

Unlock Deck
Unlock for access to all 39 flashcards in this deck.
Unlock Deck
k this deck
13
Which amino acid does not have an enantiomer?
A) Alanine
B) Glutamine
C) Histidine
D) Aspartic acid
E) Glycine
A) Alanine
B) Glutamine
C) Histidine
D) Aspartic acid
E) Glycine

Unlock Deck
Unlock for access to all 39 flashcards in this deck.
Unlock Deck
k this deck
14
How many tripeptides containing one residue each of L-alanine,L-valine,and glycine are possible?
A) 2
B) 3
C) 4
D) 6
E) 9
A) 2
B) 3
C) 4
D) 6
E) 9

Unlock Deck
Unlock for access to all 39 flashcards in this deck.
Unlock Deck
k this deck
15
In a DNA molecule,at which point would the base shown be attached to a deoxyribose fragment? 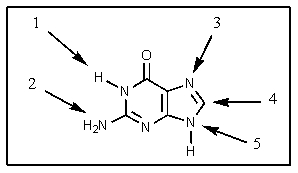
A) 1
B) 2
C) 3
D) 4
E) 5

A) 1
B) 2
C) 3
D) 4
E) 5

Unlock Deck
Unlock for access to all 39 flashcards in this deck.
Unlock Deck
k this deck
16
Chymotrypsin catalyzes hydrolysis of peptide bonds where? 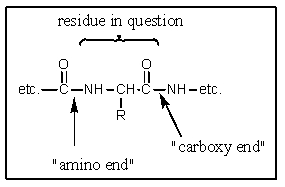
A) At the carboxy end of residues with basic side chains
B) At the amino end of residues containing aliphatic residues
C) At the carboxy end of residues with aromatic side chains
D) At the amino end of residues with aromatic side chains
E) At the carboxy end of methionine residues

A) At the carboxy end of residues with basic side chains
B) At the amino end of residues containing aliphatic residues
C) At the carboxy end of residues with aromatic side chains
D) At the amino end of residues with aromatic side chains
E) At the carboxy end of methionine residues

Unlock Deck
Unlock for access to all 39 flashcards in this deck.
Unlock Deck
k this deck
17
The artificial sweetener aspartame is a derivative of the dipeptide asp-phe.If aspartame (asp-phe)was prepared from racemic amino acids,how many stereoisomers (diastereomers + enantiomers)could be formed? 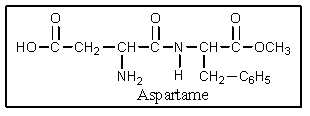
A) 2
B) 3
C) 4
D) 6
E) 8

A) 2
B) 3
C) 4
D) 6
E) 8

Unlock Deck
Unlock for access to all 39 flashcards in this deck.
Unlock Deck
k this deck
18
How many amino acid residues are present in the following polypeptide? 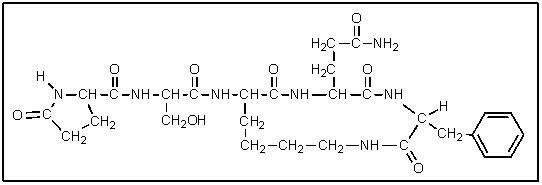
A) 4
B) 5
C) 6
D) 7
E) 8

A) 4
B) 5
C) 6
D) 7
E) 8

Unlock Deck
Unlock for access to all 39 flashcards in this deck.
Unlock Deck
k this deck
19
Which amino acid does not form a characteristic blue color with ninhydrin?
A) Cysteine
B) Lysine
C) Proline
D) Valine
E) Tryptophan
A) Cysteine
B) Lysine
C) Proline
D) Valine
E) Tryptophan

Unlock Deck
Unlock for access to all 39 flashcards in this deck.
Unlock Deck
k this deck
20
It was not until 1974 that -carboxyglutamic acid (shown below)was discovered (by researchers at the University of Colorado)in polypeptides.Based on your knowledge of organic chemistry,what is the most likely reason that this particular amino acid escaped identification so long? 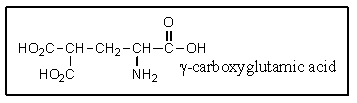
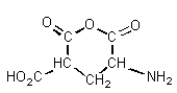
A) It tended to spontaneously form a cyclic anhydride:
B) It tended to spontaneously lose carbon dioxide:
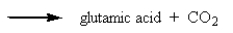
C) It easily underwent a reverse-Knovenagle reaction:
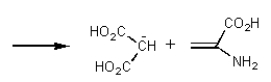
D) It tended to spontaneously form a cyclic amide (lactam):
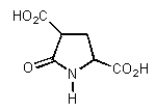
E) It was simply too polar to extract into non-polar organic solvents.

A) It tended to spontaneously form a cyclic anhydride:

B) It tended to spontaneously lose carbon dioxide:
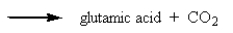
C) It easily underwent a reverse-Knovenagle reaction:

D) It tended to spontaneously form a cyclic amide (lactam):

E) It was simply too polar to extract into non-polar organic solvents.

Unlock Deck
Unlock for access to all 39 flashcards in this deck.
Unlock Deck
k this deck
21
The secondary structure of proteins is held together by hydrogen bonds between which protein sub-structures?
A) The C=O and N-H on amino acid side chains
B) The C=O on the protein backbone and the N-H on the amino acid side chains
C) The C=O on the amino acid side chains and the N-H on the protein backbone
D) The C=O and the N-H on the protein backbone
E) There are no hydrogen bonds involved with secondary structure.
A) The C=O and N-H on amino acid side chains
B) The C=O on the protein backbone and the N-H on the amino acid side chains
C) The C=O on the amino acid side chains and the N-H on the protein backbone
D) The C=O and the N-H on the protein backbone
E) There are no hydrogen bonds involved with secondary structure.

Unlock Deck
Unlock for access to all 39 flashcards in this deck.
Unlock Deck
k this deck
22
Which amino acid is shown? 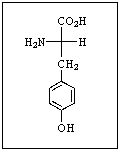
A) Alanine
B) Glycine
C) Tyrosine
D) Lysine
E) Arginine

A) Alanine
B) Glycine
C) Tyrosine
D) Lysine
E) Arginine

Unlock Deck
Unlock for access to all 39 flashcards in this deck.
Unlock Deck
k this deck
23
Which of the following DNA base pairs can participate in Watson and Crick type hydrogen bonding?
A) A-T
B) G-T
C) G-C
D) Both A and B
E) Both A and C
A) A-T
B) G-T
C) G-C
D) Both A and B
E) Both A and C

Unlock Deck
Unlock for access to all 39 flashcards in this deck.
Unlock Deck
k this deck
24
Which attractive force is responsible for maintaining the tertiary structure of proteins?
A) Disulfide linkages
B) Hydrogen bonds
C) Salt bridges
D) Hydrophobic interactions
E) All of these
A) Disulfide linkages
B) Hydrogen bonds
C) Salt bridges
D) Hydrophobic interactions
E) All of these

Unlock Deck
Unlock for access to all 39 flashcards in this deck.
Unlock Deck
k this deck
25
The difference(s)between DNA and RNA is (are):
A) DNA incorporates 2-deoxyribose sugars rather than ribose.
B) RNA is smaller than DNA.
C) DNA uses thymine,RNA uses uracil.
D) Two of the above are true.
E) All of the above are true.
A) DNA incorporates 2-deoxyribose sugars rather than ribose.
B) RNA is smaller than DNA.
C) DNA uses thymine,RNA uses uracil.
D) Two of the above are true.
E) All of the above are true.

Unlock Deck
Unlock for access to all 39 flashcards in this deck.
Unlock Deck
k this deck
26
At the isoelectric point,the amino acid alanine will exist as what structure?
A) A positive ion
B) A negative ion
C) An uncharged molecule
D) A zwitterion
E) A carbocation
A) A positive ion
B) A negative ion
C) An uncharged molecule
D) A zwitterion
E) A carbocation

Unlock Deck
Unlock for access to all 39 flashcards in this deck.
Unlock Deck
k this deck
27
Which of the following amino acids contains an aromatic ring?
A) Alanine
B) Serine
C) Tyrosine
D) Asparagine
E) Glycine
A) Alanine
B) Serine
C) Tyrosine
D) Asparagine
E) Glycine

Unlock Deck
Unlock for access to all 39 flashcards in this deck.
Unlock Deck
k this deck
28
Which amino acid is responsible for the condition known as phenylketonuria (PKU)which occurs in a small fraction of the population?
A) Tryptophan
B) Histidine
C) Phenylalanine
D) Proline
E) Valine
A) Tryptophan
B) Histidine
C) Phenylalanine
D) Proline
E) Valine

Unlock Deck
Unlock for access to all 39 flashcards in this deck.
Unlock Deck
k this deck
29
What process would one use to make multiple copies of a fragment of DNA?
A) Polymerase chain reaction
B) Electrophoresis
C) Transcription
D) Translation
E) Merrifield solid-phase synthesis
A) Polymerase chain reaction
B) Electrophoresis
C) Transcription
D) Translation
E) Merrifield solid-phase synthesis

Unlock Deck
Unlock for access to all 39 flashcards in this deck.
Unlock Deck
k this deck
30
Amino acids are covalently bonded to each other through_______.
A) hydrogen bonding
B) disulfide bridges
C) peptide bonds
D) ( and folds)
E) None of the above.
A) hydrogen bonding
B) disulfide bridges
C) peptide bonds
D) ( and folds)
E) None of the above.

Unlock Deck
Unlock for access to all 39 flashcards in this deck.
Unlock Deck
k this deck
31
The organic portion of which of the following biological materials does not consist mainly of polypeptides and/or proteins?
A) Enzymes
B) Hair
C) Hemoglobin
D) Membranes
E) Insulin
A) Enzymes
B) Hair
C) Hemoglobin
D) Membranes
E) Insulin

Unlock Deck
Unlock for access to all 39 flashcards in this deck.
Unlock Deck
k this deck
32
Although traces of racemic amino acids have been found in meteorites,nearly all naturally occurring amino acids on Earth have the stereochemistry shown.What is true of this stereochemistry? (Assume R = alkyl) 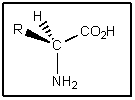
A) Amino acids are of the (R)configuration.
B) Amino acids are of the (S)configuration.
C) Amino acids are not chiral.
D) Amino acids are meso compounds.
E) Amino acids easily change configuration.

A) Amino acids are of the (R)configuration.
B) Amino acids are of the (S)configuration.
C) Amino acids are not chiral.
D) Amino acids are meso compounds.
E) Amino acids easily change configuration.

Unlock Deck
Unlock for access to all 39 flashcards in this deck.
Unlock Deck
k this deck
33
Folding of polypeptides influenced by distant residues is an example of what protein structure?
A) Primary
B) Secondary
C) Tertiary
D) Quaternary
E) None of the above.
A) Primary
B) Secondary
C) Tertiary
D) Quaternary
E) None of the above.

Unlock Deck
Unlock for access to all 39 flashcards in this deck.
Unlock Deck
k this deck
34
Which of the following represents how a typical amino acid would mainly exist in basic solution (pH > 7)?
A)
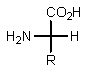
B)
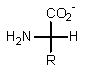
C)
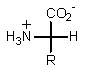
D)
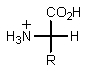
E) None of the above.
A)

B)

C)
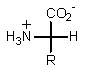
D)
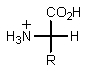
E) None of the above.

Unlock Deck
Unlock for access to all 39 flashcards in this deck.
Unlock Deck
k this deck
35
How many polypeptides would be present if the following was treated with trypsin? 
A) 1
B) 2
C) 3
D) 4
E) 5
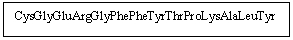
A) 1
B) 2
C) 3
D) 4
E) 5

Unlock Deck
Unlock for access to all 39 flashcards in this deck.
Unlock Deck
k this deck
36
Which nitrogen in the imidazole ring of histidine is the more basic? 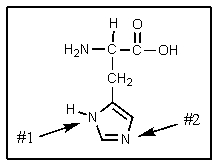
A) #1
B) #2
C) both have same basicity
D) neither is basic
E) the histidine N-H is actually acidic

A) #1
B) #2
C) both have same basicity
D) neither is basic
E) the histidine N-H is actually acidic

Unlock Deck
Unlock for access to all 39 flashcards in this deck.
Unlock Deck
k this deck
37
Which of the following statements is not true of amino acids?
A) They are less acidic than carboxylic acids.
B) In acid solution (pH ~ 2)they would show an IR absorption near 1720 cm-1.
C) They are soluble in water but not in non-polar organic solvents.
D) They are more basic than amines.
E) In basic solution (pH ~ 12)they would show an IR absorption near 3300 cm-1.
A) They are less acidic than carboxylic acids.
B) In acid solution (pH ~ 2)they would show an IR absorption near 1720 cm-1.
C) They are soluble in water but not in non-polar organic solvents.
D) They are more basic than amines.
E) In basic solution (pH ~ 12)they would show an IR absorption near 3300 cm-1.

Unlock Deck
Unlock for access to all 39 flashcards in this deck.
Unlock Deck
k this deck
38
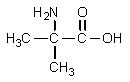
What would result from the following reactions? 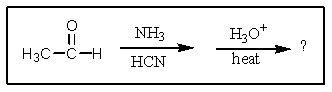
A)
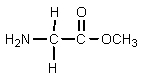
B)
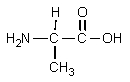
C)
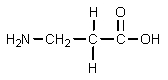
D)
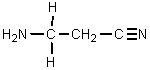
E)

A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 39 flashcards in this deck.
Unlock Deck
k this deck
39
Given that the pKa of the acidic form of proline is 2.0,and the pKa of the basic form of proline is 10.6,what is the isoelectric point?
A) 2.0
B) 6.3
C) 4.4
D) 10.6
E) 7.0
A) 2.0
B) 6.3
C) 4.4
D) 10.6
E) 7.0

Unlock Deck
Unlock for access to all 39 flashcards in this deck.
Unlock Deck
k this deck



