Deck 25: Heterocycles: Heteroatoms in Cyclic Organic Compounds
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/23
Play
Full screen (f)
Deck 25: Heterocycles: Heteroatoms in Cyclic Organic Compounds
1
Predict the major product of the following reaction. 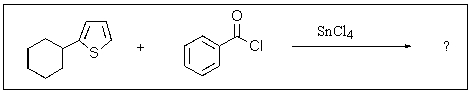
A)
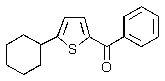
B)
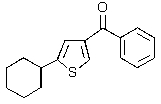
C)

D)
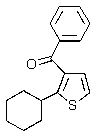
E)
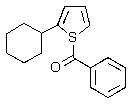

A)

B)

C)

D)

E)


2
What product(s)would you obtain from the reaction below? 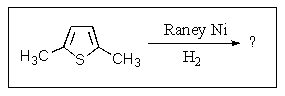
A)
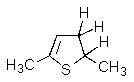
B)
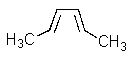
C)
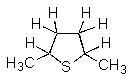
D)

E)
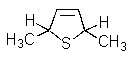

A)

B)

C)

D)

E)


3
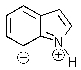
Indole is stabilized by several charge-separated resonance contributors.Which of the following is not a contributing charge-separated resonance structure? 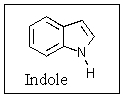
A)
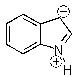
B)
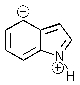
C)
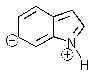
D)
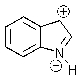
E)

A)

B)

C)

D)

E)


4
Which atom in the following heterocycle would be the most basic? 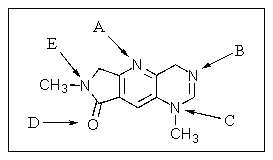
A) A
B) B
C) C
D) D
E) E

A) A
B) B
C) C
D) D
E) E

Unlock Deck
Unlock for access to all 23 flashcards in this deck.
Unlock Deck
k this deck
5
Which of the following would be the most basic?
A)
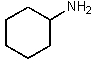
B)
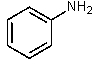
C)
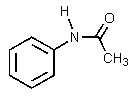
D)
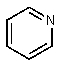
E)
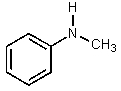
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 23 flashcards in this deck.
Unlock Deck
k this deck
6
What is the name of the following amine? 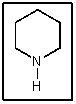
A) Piperidine
B) Pyrrolidine
C) Morpholine
D) Piperazine
E) Pyridine

A) Piperidine
B) Pyrrolidine
C) Morpholine
D) Piperazine
E) Pyridine

Unlock Deck
Unlock for access to all 23 flashcards in this deck.
Unlock Deck
k this deck
7
Which of the following would be the most reactive diene in a Diels-Alder reaction?
A)
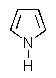
B)
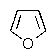
C)
D) These are equally reactive.
E) None of these undergo Diels-Alder reactions.
A)

B)

C)

D) These are equally reactive.
E) None of these undergo Diels-Alder reactions.

Unlock Deck
Unlock for access to all 23 flashcards in this deck.
Unlock Deck
k this deck
8
Which of the chloroquinolines shown below would be most reactive toward sodium methoxide?
A)
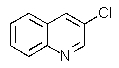
B)
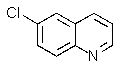
C)
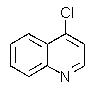
D)
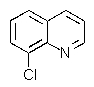
E) These are expected to be equally reactive.
A)

B)

C)

D)

E) These are expected to be equally reactive.

Unlock Deck
Unlock for access to all 23 flashcards in this deck.
Unlock Deck
k this deck
9
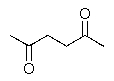
What product would be formed in the reaction below? 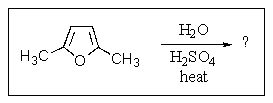
A)
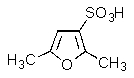
B)
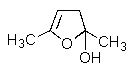
C)
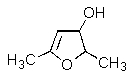
D)
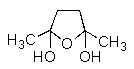
E)

A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 23 flashcards in this deck.
Unlock Deck
k this deck
10
Which reagent might convert 2,5-hexanedione to 2,5-dimethylfuran? 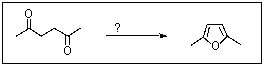
A) NaBH4,CH3OH
B) H2,Pt
C) NaOH,heat
D) P2O5
E) Na,EtOH

A) NaBH4,CH3OH
B) H2,Pt
C) NaOH,heat
D) P2O5
E) Na,EtOH

Unlock Deck
Unlock for access to all 23 flashcards in this deck.
Unlock Deck
k this deck
11
How would the heterocyclic amines shown here be ranked in order of decreasing basicity (more basic > less basic)? 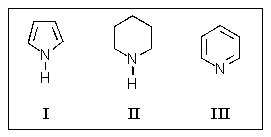
A) III > I > II
B) I > II > III
C) II > III > I
D) I > III > II
E) II > I > III

A) III > I > II
B) I > II > III
C) II > III > I
D) I > III > II
E) II > I > III

Unlock Deck
Unlock for access to all 23 flashcards in this deck.
Unlock Deck
k this deck
12
Which of the following is not formed as either an intermediate along the reaction pathway or as a final product for the Chichibabin reaction shown below? 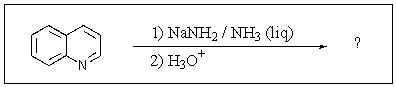
A)
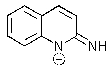
B)
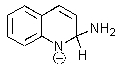
C)
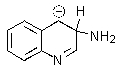
D)
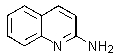
E)
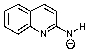
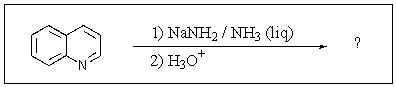
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 23 flashcards in this deck.
Unlock Deck
k this deck
13
The best way to prepare a 2-alkylpyridine from pyridine would be what? 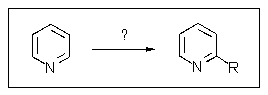
A) Electrophilic substitution
B) Free-radical substitution
C) Nucleophilic substitution
D) An SN2 reaction
E) None of the above are correct.

A) Electrophilic substitution
B) Free-radical substitution
C) Nucleophilic substitution
D) An SN2 reaction
E) None of the above are correct.

Unlock Deck
Unlock for access to all 23 flashcards in this deck.
Unlock Deck
k this deck
14
What product do you expect from the reaction shown below? 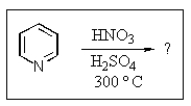
A)
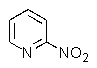
B)
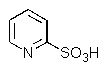
C)
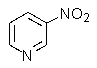
D)
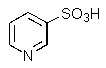
E)
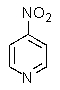

A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 23 flashcards in this deck.
Unlock Deck
k this deck
15
Which of the following structures represents a quinoline derivative?
A)
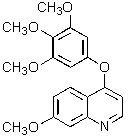
B)
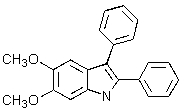
C)
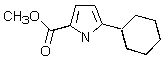
D)
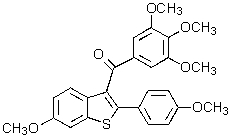
E)
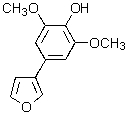
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 23 flashcards in this deck.
Unlock Deck
k this deck
16
Which of the following is not aromatic?
A)
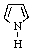
B)
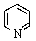
C)
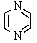
D)
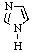
E) All are aromatic.
A)
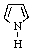
B)
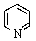
C)
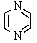
D)
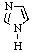
E) All are aromatic.

Unlock Deck
Unlock for access to all 23 flashcards in this deck.
Unlock Deck
k this deck
17
Which nitrogen,if either,is the more basic in nicotine? 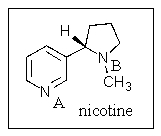
A) Nitrogen A is more basic.
B) Nitrogen B is more basic.
C) The nitrogens are equally basic.
D) Neither nitrogen is basic.
E) There is no basis on which to predict this.

A) Nitrogen A is more basic.
B) Nitrogen B is more basic.
C) The nitrogens are equally basic.
D) Neither nitrogen is basic.
E) There is no basis on which to predict this.

Unlock Deck
Unlock for access to all 23 flashcards in this deck.
Unlock Deck
k this deck
18
Predict the major product of the following reaction. 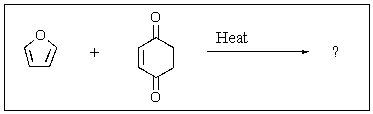
A)
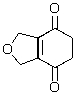
B)
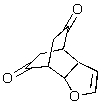
C)
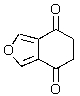
D)
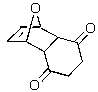
E)
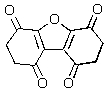

A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 23 flashcards in this deck.
Unlock Deck
k this deck
19
You would expect a very strong nucleophile (e.g.,H2N-)to attack 2-methylpyridine where? 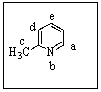
A) Position a
B) Position b
C) Position c
D) Position d
E) Position e

A) Position a
B) Position b
C) Position c
D) Position d
E) Position e

Unlock Deck
Unlock for access to all 23 flashcards in this deck.
Unlock Deck
k this deck
20
You would expect a powerful electrophile (e.g.,Br+)to attack 2-methylpyridine mostly at which carbon? 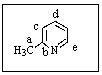
A) Position a
B) Position b
C) Position c
D) Position d
E) Position e

A) Position a
B) Position b
C) Position c
D) Position d
E) Position e

Unlock Deck
Unlock for access to all 23 flashcards in this deck.
Unlock Deck
k this deck
21
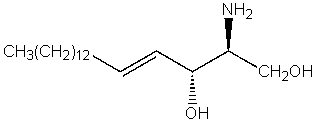
Which of the following compounds is not a heterocycle?
A)
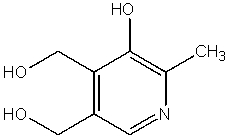
B)
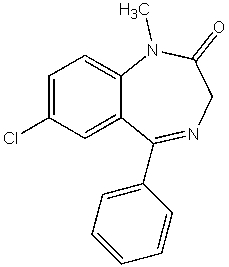
C)
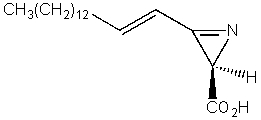
D)
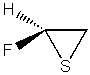
E)
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 23 flashcards in this deck.
Unlock Deck
k this deck
22
What is the product of the following reaction? 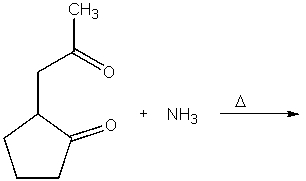
A)
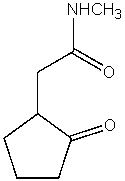
B)
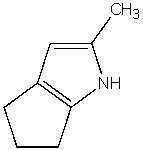
C)
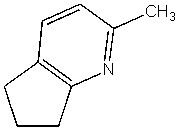
D)
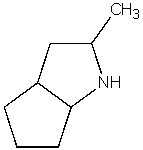
E) None of the above.

A)

B)

C)

D)

E) None of the above.

Unlock Deck
Unlock for access to all 23 flashcards in this deck.
Unlock Deck
k this deck
23
Which of the following compounds is not an alkaloid?
A)
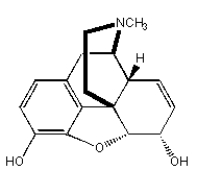
B)
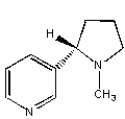
C)
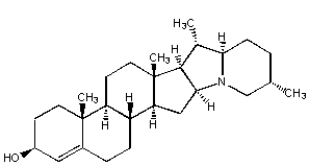
D)
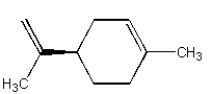
E) All of the above are alkaloids.
A)

B)

C)

D)

E) All of the above are alkaloids.

Unlock Deck
Unlock for access to all 23 flashcards in this deck.
Unlock Deck
k this deck



