Deck 26: Orbitals and Organic Chemistry: Pericyclic Reactionse
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/44
Play
Full screen (f)
Deck 26: Orbitals and Organic Chemistry: Pericyclic Reactionse
1
Exhibit 30-5
To answer the following question(s), consider the reaction below: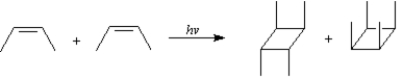
Refer to Exhibit 30-5. Propose a mechanism for the reaction that fully accounts for the formation of both products.
To answer the following question(s), consider the reaction below:

Refer to Exhibit 30-5. Propose a mechanism for the reaction that fully accounts for the formation of both products.
![This reaction occurs with suprafacial stereochemistry that would be expected for a photochemical [2 + 2] cycloaddition. However, suprafacial cycloaddition can occur in two different ways⎯with the methyl groups of both alkenes on the same side or on opposite sides.](https://d2lvgg3v3hfg70.cloudfront.net/TB6688/11eab600_07e3_8e0d_9625_03d9c9b780f5_TB6688_00.jpg) This reaction occurs with suprafacial stereochemistry that would be expected for a photochemical [2 + 2] cycloaddition. However, suprafacial cycloaddition can occur in two different ways⎯with the methyl groups of both alkenes on the same side or on opposite sides.
This reaction occurs with suprafacial stereochemistry that would be expected for a photochemical [2 + 2] cycloaddition. However, suprafacial cycloaddition can occur in two different ways⎯with the methyl groups of both alkenes on the same side or on opposite sides. 2
To answer the following question(s), consider the π molecular orbitals of a conjugated diene shown below: 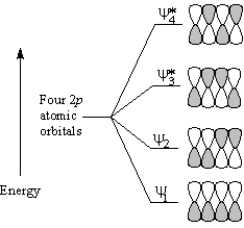
Refer to Exhibit 30-1. Which molecular orbital of a ground-state conjugated diene is the highest occupied molecular orbital (HOMO)?
A) ψ1
B) ψ2
C) ψ3*
D) ψ4*

Refer to Exhibit 30-1. Which molecular orbital of a ground-state conjugated diene is the highest occupied molecular orbital (HOMO)?
A) ψ1
B) ψ2
C) ψ3*
D) ψ4*
ψ2
3
Exhibit 30-3
To answer the following question(s), consider the reaction below: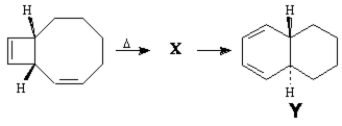
Refer to Exhibit 30-3. Explain the stereochemistry of compound Y.
To answer the following question(s), consider the reaction below:

Refer to Exhibit 30-3. Explain the stereochemistry of compound Y.
Thermal electrocyclic opening of the cis-substituted cyclobutene yields (1E, 3Z, 5Z)-cyclodecatriene (X). Symmetry-allowed thermal cyclization of the cyclodecatriene occurs in a disrotatory fashion to yield the observed compound Y. 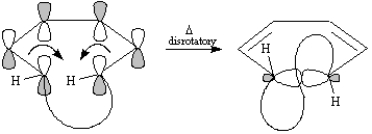

4
Exhibit 30-2
Consider the reaction below to answer the following question(s):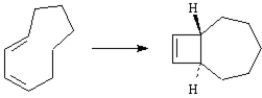
Refer to Exhibit 30-2. Has this reaction taken place in a conrotatory manner or in a disrotatory manner?
Consider the reaction below to answer the following question(s):

Refer to Exhibit 30-2. Has this reaction taken place in a conrotatory manner or in a disrotatory manner?

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
5
To answer the following question(s), consider the π molecular orbitals of a conjugated diene shown below: 
Refer to Exhibit 30-1. Which molecular orbital of an excited-state conjugated diene is the lowest unoccupied molecular orbital (LUMO)?
A) ψ1
B) ψ2
C) ψ3*
D) ψ4*

Refer to Exhibit 30-1. Which molecular orbital of an excited-state conjugated diene is the lowest unoccupied molecular orbital (LUMO)?
A) ψ1
B) ψ2
C) ψ3*
D) ψ4*

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
6
Exhibit 30-6
Consider the reaction sequence below to answer the following question(s):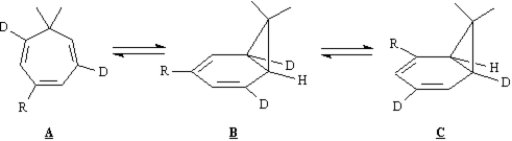
Refer to Exhibit 30-6. Explain the stereochemistry of product B.
Consider the reaction sequence below to answer the following question(s):

Refer to Exhibit 30-6. Explain the stereochemistry of product B.

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
7
Exhibit 30-5
To answer the following question(s), consider the reaction below:
Refer to Exhibit 30-5. How many pairs of electrons are involved in this pericyclic reaction?
A) two
B) four
C) eight
D) sixteen
To answer the following question(s), consider the reaction below:

Refer to Exhibit 30-5. How many pairs of electrons are involved in this pericyclic reaction?
A) two
B) four
C) eight
D) sixteen

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
8
To answer the following question(s), consider the π molecular orbitals of a conjugated diene shown below: 
Draw the product(s) you would expect to obtain from photochemical cyclization of (2Z, 4E)-hexadiene.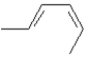

Draw the product(s) you would expect to obtain from photochemical cyclization of (2Z, 4E)-hexadiene.


Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
9
Exhibit 30-4
Consider the reaction below to answer the following question(s):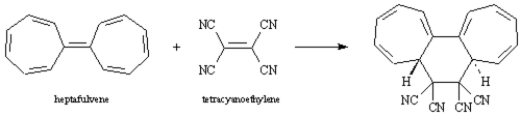
Refer to Exhibit 30-4. Would you expect this reaction to occur under photochemical or thermal conditions?
Consider the reaction below to answer the following question(s):

Refer to Exhibit 30-4. Would you expect this reaction to occur under photochemical or thermal conditions?

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
10
To answer the following question(s), consider the π molecular orbitals of a conjugated diene shown below: 
Refer to Exhibit 30-1. Which molecular orbital of a conjugated diene contains two nodes between nuclei?
A) ψ1
B) ψ2
C) ψ3*
D) ψ4*

Refer to Exhibit 30-1. Which molecular orbital of a conjugated diene contains two nodes between nuclei?
A) ψ1
B) ψ2
C) ψ3*
D) ψ4*

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
11
Exhibit 30-2
Consider the reaction below to answer the following question(s):
Refer to Exhibit 30-2. Under what conditions, thermal or photochemical, would you carry out this reaction? Explain.
Consider the reaction below to answer the following question(s):

Refer to Exhibit 30-2. Under what conditions, thermal or photochemical, would you carry out this reaction? Explain.

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
12
Exhibit 30-3
To answer the following question(s), consider the reaction below:
Refer to Exhibit 30-3. The thermal reaction which generates X is an example of:
A) an electrocyclic reaction
B) a sigmatropic rearrangement
C) a reverse cycloaddition reaction
D) a cycloaddition reaction
To answer the following question(s), consider the reaction below:

Refer to Exhibit 30-3. The thermal reaction which generates X is an example of:
A) an electrocyclic reaction
B) a sigmatropic rearrangement
C) a reverse cycloaddition reaction
D) a cycloaddition reaction

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
13
Exhibit 30-4
Consider the reaction below to answer the following question(s):
Refer to Exhibit 30-4. What type of pericyclic reaction is involved in this transformation?
A) sigmatropic rearrangement
B) reverse cycloaddition reaction
C) electrocyclic reaction
D) cycloaddition reaction
Consider the reaction below to answer the following question(s):

Refer to Exhibit 30-4. What type of pericyclic reaction is involved in this transformation?
A) sigmatropic rearrangement
B) reverse cycloaddition reaction
C) electrocyclic reaction
D) cycloaddition reaction

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
14
Exhibit 30-5
To answer the following question(s), consider the reaction below:
Refer to Exhibit 30-5. What type of pericyclic reaction is involved in this transformation?
A) sigmatropic rearrangement
B) reverse cycloaddition reaction
C) electrocyclic reaction
D) cycloaddition reaction
To answer the following question(s), consider the reaction below:

Refer to Exhibit 30-5. What type of pericyclic reaction is involved in this transformation?
A) sigmatropic rearrangement
B) reverse cycloaddition reaction
C) electrocyclic reaction
D) cycloaddition reaction

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
15
Exhibit 30-3
To answer the following question(s), consider the reaction below:
Refer to Exhibit 30-3. Two pericyclic reactions are involved in this synthesis of compound Y. Draw the structure of intermediate X and name it using IUPAC nomenclature.
To answer the following question(s), consider the reaction below:

Refer to Exhibit 30-3. Two pericyclic reactions are involved in this synthesis of compound Y. Draw the structure of intermediate X and name it using IUPAC nomenclature.

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
16
Exhibit 30-4
Consider the reaction below to answer the following question(s):
Refer to Exhibit 30-4. How many pairs of electrons are involved in this pericyclic reaction?
A) two
B) four
C) eight
D) sixteen
Consider the reaction below to answer the following question(s):

Refer to Exhibit 30-4. How many pairs of electrons are involved in this pericyclic reaction?
A) two
B) four
C) eight
D) sixteen

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
17
Exhibit 30-6
Consider the reaction sequence below to answer the following question(s):
Refer to Exhibit 30-6. Step 1 is an example of:
A) an electrocyclic reaction
B) a cycloaddition reaction
C) a sigmatropic rearrangement
D) a reverse cycloaddition reaction
Consider the reaction sequence below to answer the following question(s):

Refer to Exhibit 30-6. Step 1 is an example of:
A) an electrocyclic reaction
B) a cycloaddition reaction
C) a sigmatropic rearrangement
D) a reverse cycloaddition reaction

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
18
The mnemonic phrase "The Electrons Circle Around", TECA, assists in predicting stereochemistry of pericyclic reactions. Describe application of this mnemonic.

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
19
Exhibit 30-4
Consider the reaction below to answer the following question(s):
Refer to Exhibit 30-4. Explain the stereochemistry of this reaction.
Consider the reaction below to answer the following question(s):

Refer to Exhibit 30-4. Explain the stereochemistry of this reaction.

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
20
There are three major classes of pericyclic reactions. Which of the following types of reactions is not an example of a pericyclic process?
A) sigmatropic rearrangements
B) cycloaddition reactions
C) annulation reactions
D) electrocyclic reactions
A) sigmatropic rearrangements
B) cycloaddition reactions
C) annulation reactions
D) electrocyclic reactions

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
21
Define a pericyclic reaction.

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
22
The photochemical cycloaddition of the structure below is related to DNA mutations causing skin cancer. Draw the structure of this dimer. Atoms other than carbon and hydrogen are labeled. 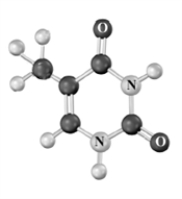


Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
23
Exhibit 30-8
Classify each of the following sigmatropic reactions by order [x, y].
26.![<strong>Exhibit 30-8 Classify each of the following sigmatropic reactions by order [x, y]. 26. ANSWER: [3, 3] POINTS: 1 27. ANSWER: [1, 7] POINTS: 1 28. ANSWER: [1, 5] POINTS: 1 Exhibit 30-9 Vitamin D<sub>3</sub>, cholecalciferol, is synthesized under the skin by the photochemical pericyclic process shown below. Answer the following question(s) about these reactions. Refer to Exhibit 30-9. The second step in this process is a(n):</strong> A) sigmatropic rearrangement B) cycloaddition reaction C) annulation reaction D) electrocyclic reaction](https://d2lvgg3v3hfg70.cloudfront.net/TB6688/11eab600_07e4_9f81_9625_9375e065cacf_TB6688_00_TB6688_00.jpg) ANSWER:
ANSWER:
[3, 3]![<strong>Exhibit 30-8 Classify each of the following sigmatropic reactions by order [x, y]. 26. ANSWER: [3, 3] POINTS: 1 27. ANSWER: [1, 7] POINTS: 1 28. ANSWER: [1, 5] POINTS: 1 Exhibit 30-9 Vitamin D<sub>3</sub>, cholecalciferol, is synthesized under the skin by the photochemical pericyclic process shown below. Answer the following question(s) about these reactions. Refer to Exhibit 30-9. The second step in this process is a(n):</strong> A) sigmatropic rearrangement B) cycloaddition reaction C) annulation reaction D) electrocyclic reaction](https://d2lvgg3v3hfg70.cloudfront.net/TB6688/11eab600_07e4_9f82_9625_478e7c8399a6_TB6688_00_TB6688_00.jpg) POINTS:
POINTS:
1
27.![<strong>Exhibit 30-8 Classify each of the following sigmatropic reactions by order [x, y]. 26. ANSWER: [3, 3] POINTS: 1 27. ANSWER: [1, 7] POINTS: 1 28. ANSWER: [1, 5] POINTS: 1 Exhibit 30-9 Vitamin D<sub>3</sub>, cholecalciferol, is synthesized under the skin by the photochemical pericyclic process shown below. Answer the following question(s) about these reactions. Refer to Exhibit 30-9. The second step in this process is a(n):</strong> A) sigmatropic rearrangement B) cycloaddition reaction C) annulation reaction D) electrocyclic reaction](https://d2lvgg3v3hfg70.cloudfront.net/TB6688/11eab600_07e4_c693_9625_e7ab50d9cadf_TB6688_00_TB6688_00.jpg) ANSWER:
ANSWER:
[1, 7]![<strong>Exhibit 30-8 Classify each of the following sigmatropic reactions by order [x, y]. 26. ANSWER: [3, 3] POINTS: 1 27. ANSWER: [1, 7] POINTS: 1 28. ANSWER: [1, 5] POINTS: 1 Exhibit 30-9 Vitamin D<sub>3</sub>, cholecalciferol, is synthesized under the skin by the photochemical pericyclic process shown below. Answer the following question(s) about these reactions. Refer to Exhibit 30-9. The second step in this process is a(n):</strong> A) sigmatropic rearrangement B) cycloaddition reaction C) annulation reaction D) electrocyclic reaction](https://d2lvgg3v3hfg70.cloudfront.net/TB6688/11eab600_07e4_c694_9625_452e3d23b69b_TB6688_00_TB6688_00.jpg) POINTS:
POINTS:
1
28.![<strong>Exhibit 30-8 Classify each of the following sigmatropic reactions by order [x, y]. 26. ANSWER: [3, 3] POINTS: 1 27. ANSWER: [1, 7] POINTS: 1 28. ANSWER: [1, 5] POINTS: 1 Exhibit 30-9 Vitamin D<sub>3</sub>, cholecalciferol, is synthesized under the skin by the photochemical pericyclic process shown below. Answer the following question(s) about these reactions. Refer to Exhibit 30-9. The second step in this process is a(n):</strong> A) sigmatropic rearrangement B) cycloaddition reaction C) annulation reaction D) electrocyclic reaction](https://d2lvgg3v3hfg70.cloudfront.net/TB6688/11eab600_07e4_c695_9625_3f0a2cdc4491_TB6688_00_TB6688_00.jpg) ANSWER:
ANSWER:
[1, 5]![<strong>Exhibit 30-8 Classify each of the following sigmatropic reactions by order [x, y]. 26. ANSWER: [3, 3] POINTS: 1 27. ANSWER: [1, 7] POINTS: 1 28. ANSWER: [1, 5] POINTS: 1 Exhibit 30-9 Vitamin D<sub>3</sub>, cholecalciferol, is synthesized under the skin by the photochemical pericyclic process shown below. Answer the following question(s) about these reactions. Refer to Exhibit 30-9. The second step in this process is a(n):</strong> A) sigmatropic rearrangement B) cycloaddition reaction C) annulation reaction D) electrocyclic reaction](https://d2lvgg3v3hfg70.cloudfront.net/TB6688/11eab600_07e4_eda6_9625_178a2380404f_TB6688_00_TB6688_00.jpg) POINTS:
POINTS:
1
Exhibit 30-9
Vitamin D3, cholecalciferol, is synthesized under the skin by the photochemical pericyclic process shown below. Answer the following question(s) about these reactions.![<strong>Exhibit 30-8 Classify each of the following sigmatropic reactions by order [x, y]. 26. ANSWER: [3, 3] POINTS: 1 27. ANSWER: [1, 7] POINTS: 1 28. ANSWER: [1, 5] POINTS: 1 Exhibit 30-9 Vitamin D<sub>3</sub>, cholecalciferol, is synthesized under the skin by the photochemical pericyclic process shown below. Answer the following question(s) about these reactions. Refer to Exhibit 30-9. The second step in this process is a(n):</strong> A) sigmatropic rearrangement B) cycloaddition reaction C) annulation reaction D) electrocyclic reaction](https://d2lvgg3v3hfg70.cloudfront.net/TB6688/11eab600_07e4_eda7_9625_2bab5519f51f_TB6688_00_TB6688_00.jpg)
Refer to Exhibit 30-9. The second step in this process is a(n):
A) sigmatropic rearrangement
B) cycloaddition reaction
C) annulation reaction
D) electrocyclic reaction
Classify each of the following sigmatropic reactions by order [x, y].
26.
![<strong>Exhibit 30-8 Classify each of the following sigmatropic reactions by order [x, y]. 26. ANSWER: [3, 3] POINTS: 1 27. ANSWER: [1, 7] POINTS: 1 28. ANSWER: [1, 5] POINTS: 1 Exhibit 30-9 Vitamin D<sub>3</sub>, cholecalciferol, is synthesized under the skin by the photochemical pericyclic process shown below. Answer the following question(s) about these reactions. Refer to Exhibit 30-9. The second step in this process is a(n):</strong> A) sigmatropic rearrangement B) cycloaddition reaction C) annulation reaction D) electrocyclic reaction](https://d2lvgg3v3hfg70.cloudfront.net/TB6688/11eab600_07e4_9f81_9625_9375e065cacf_TB6688_00_TB6688_00.jpg) ANSWER:
ANSWER:[3, 3]
![<strong>Exhibit 30-8 Classify each of the following sigmatropic reactions by order [x, y]. 26. ANSWER: [3, 3] POINTS: 1 27. ANSWER: [1, 7] POINTS: 1 28. ANSWER: [1, 5] POINTS: 1 Exhibit 30-9 Vitamin D<sub>3</sub>, cholecalciferol, is synthesized under the skin by the photochemical pericyclic process shown below. Answer the following question(s) about these reactions. Refer to Exhibit 30-9. The second step in this process is a(n):</strong> A) sigmatropic rearrangement B) cycloaddition reaction C) annulation reaction D) electrocyclic reaction](https://d2lvgg3v3hfg70.cloudfront.net/TB6688/11eab600_07e4_9f82_9625_478e7c8399a6_TB6688_00_TB6688_00.jpg) POINTS:
POINTS:1
27.
![<strong>Exhibit 30-8 Classify each of the following sigmatropic reactions by order [x, y]. 26. ANSWER: [3, 3] POINTS: 1 27. ANSWER: [1, 7] POINTS: 1 28. ANSWER: [1, 5] POINTS: 1 Exhibit 30-9 Vitamin D<sub>3</sub>, cholecalciferol, is synthesized under the skin by the photochemical pericyclic process shown below. Answer the following question(s) about these reactions. Refer to Exhibit 30-9. The second step in this process is a(n):</strong> A) sigmatropic rearrangement B) cycloaddition reaction C) annulation reaction D) electrocyclic reaction](https://d2lvgg3v3hfg70.cloudfront.net/TB6688/11eab600_07e4_c693_9625_e7ab50d9cadf_TB6688_00_TB6688_00.jpg) ANSWER:
ANSWER:[1, 7]
![<strong>Exhibit 30-8 Classify each of the following sigmatropic reactions by order [x, y]. 26. ANSWER: [3, 3] POINTS: 1 27. ANSWER: [1, 7] POINTS: 1 28. ANSWER: [1, 5] POINTS: 1 Exhibit 30-9 Vitamin D<sub>3</sub>, cholecalciferol, is synthesized under the skin by the photochemical pericyclic process shown below. Answer the following question(s) about these reactions. Refer to Exhibit 30-9. The second step in this process is a(n):</strong> A) sigmatropic rearrangement B) cycloaddition reaction C) annulation reaction D) electrocyclic reaction](https://d2lvgg3v3hfg70.cloudfront.net/TB6688/11eab600_07e4_c694_9625_452e3d23b69b_TB6688_00_TB6688_00.jpg) POINTS:
POINTS:1
28.
![<strong>Exhibit 30-8 Classify each of the following sigmatropic reactions by order [x, y]. 26. ANSWER: [3, 3] POINTS: 1 27. ANSWER: [1, 7] POINTS: 1 28. ANSWER: [1, 5] POINTS: 1 Exhibit 30-9 Vitamin D<sub>3</sub>, cholecalciferol, is synthesized under the skin by the photochemical pericyclic process shown below. Answer the following question(s) about these reactions. Refer to Exhibit 30-9. The second step in this process is a(n):</strong> A) sigmatropic rearrangement B) cycloaddition reaction C) annulation reaction D) electrocyclic reaction](https://d2lvgg3v3hfg70.cloudfront.net/TB6688/11eab600_07e4_c695_9625_3f0a2cdc4491_TB6688_00_TB6688_00.jpg) ANSWER:
ANSWER:[1, 5]
![<strong>Exhibit 30-8 Classify each of the following sigmatropic reactions by order [x, y]. 26. ANSWER: [3, 3] POINTS: 1 27. ANSWER: [1, 7] POINTS: 1 28. ANSWER: [1, 5] POINTS: 1 Exhibit 30-9 Vitamin D<sub>3</sub>, cholecalciferol, is synthesized under the skin by the photochemical pericyclic process shown below. Answer the following question(s) about these reactions. Refer to Exhibit 30-9. The second step in this process is a(n):</strong> A) sigmatropic rearrangement B) cycloaddition reaction C) annulation reaction D) electrocyclic reaction](https://d2lvgg3v3hfg70.cloudfront.net/TB6688/11eab600_07e4_eda6_9625_178a2380404f_TB6688_00_TB6688_00.jpg) POINTS:
POINTS:1
Exhibit 30-9
Vitamin D3, cholecalciferol, is synthesized under the skin by the photochemical pericyclic process shown below. Answer the following question(s) about these reactions.
![<strong>Exhibit 30-8 Classify each of the following sigmatropic reactions by order [x, y]. 26. ANSWER: [3, 3] POINTS: 1 27. ANSWER: [1, 7] POINTS: 1 28. ANSWER: [1, 5] POINTS: 1 Exhibit 30-9 Vitamin D<sub>3</sub>, cholecalciferol, is synthesized under the skin by the photochemical pericyclic process shown below. Answer the following question(s) about these reactions. Refer to Exhibit 30-9. The second step in this process is a(n):</strong> A) sigmatropic rearrangement B) cycloaddition reaction C) annulation reaction D) electrocyclic reaction](https://d2lvgg3v3hfg70.cloudfront.net/TB6688/11eab600_07e4_eda7_9625_2bab5519f51f_TB6688_00_TB6688_00.jpg)
Refer to Exhibit 30-9. The second step in this process is a(n):
A) sigmatropic rearrangement
B) cycloaddition reaction
C) annulation reaction
D) electrocyclic reaction

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
24
How many π MO's does the following compound have? 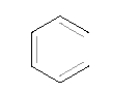
A) 2
B) 4
C) 6
D) 8

A) 2
B) 4
C) 6
D) 8

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
25
Under photochemical conditions, the following substance will undergo: 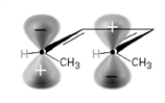
A) disrotatory cyclization.
B) conrotatory cyclization.
C) no reaction.

A) disrotatory cyclization.
B) conrotatory cyclization.
C) no reaction.

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
26
In which type of pericyclic reaction is a σ bond broken and a new σ bond formed?
A) cycloaddition
B) photochemical electrocyclic
C) thermal electrocyclic
D) sigmatropic rearrangement.
E) all of the above
A) cycloaddition
B) photochemical electrocyclic
C) thermal electrocyclic
D) sigmatropic rearrangement.
E) all of the above

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
27
Exhibit 30-8
Classify each of the following sigmatropic reactions by order [x, y].
26.![<strong>Exhibit 30-8 Classify each of the following sigmatropic reactions by order [x, y]. 26. ANSWER: [3, 3] POINTS: 1 27. ANSWER: [1, 7] POINTS: 1 28. ANSWER: [1, 5] POINTS: 1 Exhibit 30-9 Vitamin D<sub>3</sub>, cholecalciferol, is synthesized under the skin by the photochemical pericyclic process shown below. Answer the following question(s) about these reactions. Refer to Exhibit 30-9. The first step in this process is a(n):</strong> A) sigmatropic rearrangement B) cycloaddition reaction C) annulation reaction D) electrocyclic reaction](https://d2lvgg3v3hfg70.cloudfront.net/TB6688/11eab600_07e4_9f81_9625_9375e065cacf_TB6688_00_TB6688_00.jpg) ANSWER:
ANSWER:
[3, 3]![<strong>Exhibit 30-8 Classify each of the following sigmatropic reactions by order [x, y]. 26. ANSWER: [3, 3] POINTS: 1 27. ANSWER: [1, 7] POINTS: 1 28. ANSWER: [1, 5] POINTS: 1 Exhibit 30-9 Vitamin D<sub>3</sub>, cholecalciferol, is synthesized under the skin by the photochemical pericyclic process shown below. Answer the following question(s) about these reactions. Refer to Exhibit 30-9. The first step in this process is a(n):</strong> A) sigmatropic rearrangement B) cycloaddition reaction C) annulation reaction D) electrocyclic reaction](https://d2lvgg3v3hfg70.cloudfront.net/TB6688/11eab600_07e4_9f82_9625_478e7c8399a6_TB6688_00_TB6688_00.jpg) POINTS:
POINTS:
1
27.![<strong>Exhibit 30-8 Classify each of the following sigmatropic reactions by order [x, y]. 26. ANSWER: [3, 3] POINTS: 1 27. ANSWER: [1, 7] POINTS: 1 28. ANSWER: [1, 5] POINTS: 1 Exhibit 30-9 Vitamin D<sub>3</sub>, cholecalciferol, is synthesized under the skin by the photochemical pericyclic process shown below. Answer the following question(s) about these reactions. Refer to Exhibit 30-9. The first step in this process is a(n):</strong> A) sigmatropic rearrangement B) cycloaddition reaction C) annulation reaction D) electrocyclic reaction](https://d2lvgg3v3hfg70.cloudfront.net/TB6688/11eab600_07e4_c693_9625_e7ab50d9cadf_TB6688_00_TB6688_00.jpg) ANSWER:
ANSWER:
[1, 7]![<strong>Exhibit 30-8 Classify each of the following sigmatropic reactions by order [x, y]. 26. ANSWER: [3, 3] POINTS: 1 27. ANSWER: [1, 7] POINTS: 1 28. ANSWER: [1, 5] POINTS: 1 Exhibit 30-9 Vitamin D<sub>3</sub>, cholecalciferol, is synthesized under the skin by the photochemical pericyclic process shown below. Answer the following question(s) about these reactions. Refer to Exhibit 30-9. The first step in this process is a(n):</strong> A) sigmatropic rearrangement B) cycloaddition reaction C) annulation reaction D) electrocyclic reaction](https://d2lvgg3v3hfg70.cloudfront.net/TB6688/11eab600_07e4_c694_9625_452e3d23b69b_TB6688_00_TB6688_00.jpg) POINTS:
POINTS:
1
28.![<strong>Exhibit 30-8 Classify each of the following sigmatropic reactions by order [x, y]. 26. ANSWER: [3, 3] POINTS: 1 27. ANSWER: [1, 7] POINTS: 1 28. ANSWER: [1, 5] POINTS: 1 Exhibit 30-9 Vitamin D<sub>3</sub>, cholecalciferol, is synthesized under the skin by the photochemical pericyclic process shown below. Answer the following question(s) about these reactions. Refer to Exhibit 30-9. The first step in this process is a(n):</strong> A) sigmatropic rearrangement B) cycloaddition reaction C) annulation reaction D) electrocyclic reaction](https://d2lvgg3v3hfg70.cloudfront.net/TB6688/11eab600_07e4_c695_9625_3f0a2cdc4491_TB6688_00_TB6688_00.jpg) ANSWER:
ANSWER:
[1, 5]![<strong>Exhibit 30-8 Classify each of the following sigmatropic reactions by order [x, y]. 26. ANSWER: [3, 3] POINTS: 1 27. ANSWER: [1, 7] POINTS: 1 28. ANSWER: [1, 5] POINTS: 1 Exhibit 30-9 Vitamin D<sub>3</sub>, cholecalciferol, is synthesized under the skin by the photochemical pericyclic process shown below. Answer the following question(s) about these reactions. Refer to Exhibit 30-9. The first step in this process is a(n):</strong> A) sigmatropic rearrangement B) cycloaddition reaction C) annulation reaction D) electrocyclic reaction](https://d2lvgg3v3hfg70.cloudfront.net/TB6688/11eab600_07e4_eda6_9625_178a2380404f_TB6688_00_TB6688_00.jpg) POINTS:
POINTS:
1
Exhibit 30-9
Vitamin D3, cholecalciferol, is synthesized under the skin by the photochemical pericyclic process shown below. Answer the following question(s) about these reactions.![<strong>Exhibit 30-8 Classify each of the following sigmatropic reactions by order [x, y]. 26. ANSWER: [3, 3] POINTS: 1 27. ANSWER: [1, 7] POINTS: 1 28. ANSWER: [1, 5] POINTS: 1 Exhibit 30-9 Vitamin D<sub>3</sub>, cholecalciferol, is synthesized under the skin by the photochemical pericyclic process shown below. Answer the following question(s) about these reactions. Refer to Exhibit 30-9. The first step in this process is a(n):</strong> A) sigmatropic rearrangement B) cycloaddition reaction C) annulation reaction D) electrocyclic reaction](https://d2lvgg3v3hfg70.cloudfront.net/TB6688/11eab600_07e4_eda7_9625_2bab5519f51f_TB6688_00_TB6688_00.jpg)
Refer to Exhibit 30-9. The first step in this process is a(n):
A) sigmatropic rearrangement
B) cycloaddition reaction
C) annulation reaction
D) electrocyclic reaction
Classify each of the following sigmatropic reactions by order [x, y].
26.
![<strong>Exhibit 30-8 Classify each of the following sigmatropic reactions by order [x, y]. 26. ANSWER: [3, 3] POINTS: 1 27. ANSWER: [1, 7] POINTS: 1 28. ANSWER: [1, 5] POINTS: 1 Exhibit 30-9 Vitamin D<sub>3</sub>, cholecalciferol, is synthesized under the skin by the photochemical pericyclic process shown below. Answer the following question(s) about these reactions. Refer to Exhibit 30-9. The first step in this process is a(n):</strong> A) sigmatropic rearrangement B) cycloaddition reaction C) annulation reaction D) electrocyclic reaction](https://d2lvgg3v3hfg70.cloudfront.net/TB6688/11eab600_07e4_9f81_9625_9375e065cacf_TB6688_00_TB6688_00.jpg) ANSWER:
ANSWER:[3, 3]
![<strong>Exhibit 30-8 Classify each of the following sigmatropic reactions by order [x, y]. 26. ANSWER: [3, 3] POINTS: 1 27. ANSWER: [1, 7] POINTS: 1 28. ANSWER: [1, 5] POINTS: 1 Exhibit 30-9 Vitamin D<sub>3</sub>, cholecalciferol, is synthesized under the skin by the photochemical pericyclic process shown below. Answer the following question(s) about these reactions. Refer to Exhibit 30-9. The first step in this process is a(n):</strong> A) sigmatropic rearrangement B) cycloaddition reaction C) annulation reaction D) electrocyclic reaction](https://d2lvgg3v3hfg70.cloudfront.net/TB6688/11eab600_07e4_9f82_9625_478e7c8399a6_TB6688_00_TB6688_00.jpg) POINTS:
POINTS:1
27.
![<strong>Exhibit 30-8 Classify each of the following sigmatropic reactions by order [x, y]. 26. ANSWER: [3, 3] POINTS: 1 27. ANSWER: [1, 7] POINTS: 1 28. ANSWER: [1, 5] POINTS: 1 Exhibit 30-9 Vitamin D<sub>3</sub>, cholecalciferol, is synthesized under the skin by the photochemical pericyclic process shown below. Answer the following question(s) about these reactions. Refer to Exhibit 30-9. The first step in this process is a(n):</strong> A) sigmatropic rearrangement B) cycloaddition reaction C) annulation reaction D) electrocyclic reaction](https://d2lvgg3v3hfg70.cloudfront.net/TB6688/11eab600_07e4_c693_9625_e7ab50d9cadf_TB6688_00_TB6688_00.jpg) ANSWER:
ANSWER:[1, 7]
![<strong>Exhibit 30-8 Classify each of the following sigmatropic reactions by order [x, y]. 26. ANSWER: [3, 3] POINTS: 1 27. ANSWER: [1, 7] POINTS: 1 28. ANSWER: [1, 5] POINTS: 1 Exhibit 30-9 Vitamin D<sub>3</sub>, cholecalciferol, is synthesized under the skin by the photochemical pericyclic process shown below. Answer the following question(s) about these reactions. Refer to Exhibit 30-9. The first step in this process is a(n):</strong> A) sigmatropic rearrangement B) cycloaddition reaction C) annulation reaction D) electrocyclic reaction](https://d2lvgg3v3hfg70.cloudfront.net/TB6688/11eab600_07e4_c694_9625_452e3d23b69b_TB6688_00_TB6688_00.jpg) POINTS:
POINTS:1
28.
![<strong>Exhibit 30-8 Classify each of the following sigmatropic reactions by order [x, y]. 26. ANSWER: [3, 3] POINTS: 1 27. ANSWER: [1, 7] POINTS: 1 28. ANSWER: [1, 5] POINTS: 1 Exhibit 30-9 Vitamin D<sub>3</sub>, cholecalciferol, is synthesized under the skin by the photochemical pericyclic process shown below. Answer the following question(s) about these reactions. Refer to Exhibit 30-9. The first step in this process is a(n):</strong> A) sigmatropic rearrangement B) cycloaddition reaction C) annulation reaction D) electrocyclic reaction](https://d2lvgg3v3hfg70.cloudfront.net/TB6688/11eab600_07e4_c695_9625_3f0a2cdc4491_TB6688_00_TB6688_00.jpg) ANSWER:
ANSWER:[1, 5]
![<strong>Exhibit 30-8 Classify each of the following sigmatropic reactions by order [x, y]. 26. ANSWER: [3, 3] POINTS: 1 27. ANSWER: [1, 7] POINTS: 1 28. ANSWER: [1, 5] POINTS: 1 Exhibit 30-9 Vitamin D<sub>3</sub>, cholecalciferol, is synthesized under the skin by the photochemical pericyclic process shown below. Answer the following question(s) about these reactions. Refer to Exhibit 30-9. The first step in this process is a(n):</strong> A) sigmatropic rearrangement B) cycloaddition reaction C) annulation reaction D) electrocyclic reaction](https://d2lvgg3v3hfg70.cloudfront.net/TB6688/11eab600_07e4_eda6_9625_178a2380404f_TB6688_00_TB6688_00.jpg) POINTS:
POINTS:1
Exhibit 30-9
Vitamin D3, cholecalciferol, is synthesized under the skin by the photochemical pericyclic process shown below. Answer the following question(s) about these reactions.
![<strong>Exhibit 30-8 Classify each of the following sigmatropic reactions by order [x, y]. 26. ANSWER: [3, 3] POINTS: 1 27. ANSWER: [1, 7] POINTS: 1 28. ANSWER: [1, 5] POINTS: 1 Exhibit 30-9 Vitamin D<sub>3</sub>, cholecalciferol, is synthesized under the skin by the photochemical pericyclic process shown below. Answer the following question(s) about these reactions. Refer to Exhibit 30-9. The first step in this process is a(n):</strong> A) sigmatropic rearrangement B) cycloaddition reaction C) annulation reaction D) electrocyclic reaction](https://d2lvgg3v3hfg70.cloudfront.net/TB6688/11eab600_07e4_eda7_9625_2bab5519f51f_TB6688_00_TB6688_00.jpg)
Refer to Exhibit 30-9. The first step in this process is a(n):
A) sigmatropic rearrangement
B) cycloaddition reaction
C) annulation reaction
D) electrocyclic reaction

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
28
Exhibit 30-6
Consider the reaction sequence below to answer the following question(s):
Refer to Exhibit 30-6. Step 2 is an example of:
A) an electrocyclic reaction
B) a cycloaddition reaction
C) a sigmatropic rearrangement
D) a reverse cycloaddition reaction
Consider the reaction sequence below to answer the following question(s):

Refer to Exhibit 30-6. Step 2 is an example of:
A) an electrocyclic reaction
B) a cycloaddition reaction
C) a sigmatropic rearrangement
D) a reverse cycloaddition reaction

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
29
Exhibit 30-7
Consider the Sommelet-Hauser rearrangement below to answer the following question(s):![<strong>Exhibit 30-7 Consider the Sommelet-Hauser rearrangement below to answer the following question(s): Refer to Exhibit 30-7. The Sommelet-Hauser rearrangement is an example of a [2, 3] sigmatropic rearrangement. How many pairs of electrons are involved in this reaction?</strong> A) two B) three C) four D) five](https://d2lvgg3v3hfg70.cloudfront.net/TB6688/11eab600_07e4_2a50_9625_a714ab1cc28a_TB6688_00_TB6688_00.jpg)
Refer to Exhibit 30-7. The Sommelet-Hauser rearrangement is an example of a [2, 3] sigmatropic rearrangement. How many pairs of electrons are involved in this reaction?
A) two
B) three
C) four
D) five
Consider the Sommelet-Hauser rearrangement below to answer the following question(s):
![<strong>Exhibit 30-7 Consider the Sommelet-Hauser rearrangement below to answer the following question(s): Refer to Exhibit 30-7. The Sommelet-Hauser rearrangement is an example of a [2, 3] sigmatropic rearrangement. How many pairs of electrons are involved in this reaction?</strong> A) two B) three C) four D) five](https://d2lvgg3v3hfg70.cloudfront.net/TB6688/11eab600_07e4_2a50_9625_a714ab1cc28a_TB6688_00_TB6688_00.jpg)
Refer to Exhibit 30-7. The Sommelet-Hauser rearrangement is an example of a [2, 3] sigmatropic rearrangement. How many pairs of electrons are involved in this reaction?
A) two
B) three
C) four
D) five

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
30
Under thermal conditions, the following substance will undergo: 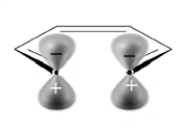
A) disrotatory cyclization.
B) conrotatory cyclization.
C) no reaction.

A) disrotatory cyclization.
B) conrotatory cyclization.
C) no reaction.

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
31
For an odd number of electron pairs (double bonds), for cycloadditions reactions which of the following rules is correct?:
A) thermal conditions - antarafacial, photochemical conditions - suprafacial
B) thermal conditions - suprafacial, photochemical conditions - antarafacial
A) thermal conditions - antarafacial, photochemical conditions - suprafacial
B) thermal conditions - suprafacial, photochemical conditions - antarafacial

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
32
Predict the product of the following reaction. 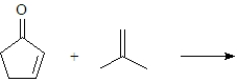


Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
33
Draw the structure of the product when the following substance reacts with maleic anhydride. Classify the reaction according to the number of π electrons that interact. Atoms other than carbon and hydrogen are labeled. 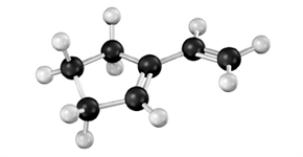


Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
34
Consider the following MO diagram for a conjugated triene. 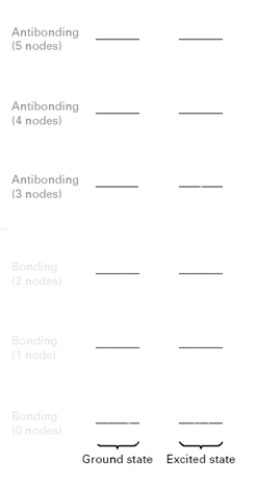 When irradiated with light of the appropriate wavelength, how many electrons will be in bonding MO's?
When irradiated with light of the appropriate wavelength, how many electrons will be in bonding MO's?
A) 6
B) 5
C) 4
D) 3
 When irradiated with light of the appropriate wavelength, how many electrons will be in bonding MO's?
When irradiated with light of the appropriate wavelength, how many electrons will be in bonding MO's?A) 6
B) 5
C) 4
D) 3

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
35
Which of the following is not an electrocyclic reaction?
A)

B)

C)
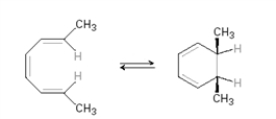
D)
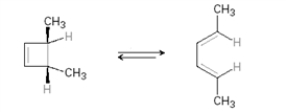
E) All are electrocyclic reactions.
A)

B)

C)

D)

E) All are electrocyclic reactions.

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
36
Exhibit 30-6
Consider the reaction sequence below to answer the following question(s):
Refer to Exhibit 30-6. Show how C is formed from B.
Consider the reaction sequence below to answer the following question(s):

Refer to Exhibit 30-6. Show how C is formed from B.

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
37
For an even number of electron pairs (double bonds), which of the following rules is correct?:
A) thermal conditions - controtatory, photochemical conditions - disrotatory
B) thermal conditions - disrotatory, photochemical conditions - conrotatory
A) thermal conditions - controtatory, photochemical conditions - disrotatory
B) thermal conditions - disrotatory, photochemical conditions - conrotatory

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
38
Exhibit 30-7
Consider the Sommelet-Hauser rearrangement below to answer the following question(s):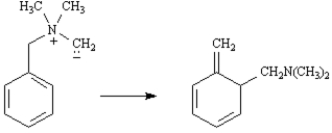
Refer to Exhibit 30-7. Would you expect the Sommelet-Hauser rearrangement to be antarafacial or suprafacial? Explain.
Consider the Sommelet-Hauser rearrangement below to answer the following question(s):

Refer to Exhibit 30-7. Would you expect the Sommelet-Hauser rearrangement to be antarafacial or suprafacial? Explain.

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
39
Exhibit 30-6
Consider the reaction sequence below to answer the following question(s):
Refer to Exhibit 30-6. Explain the stereochemistry of product C.
Consider the reaction sequence below to answer the following question(s):

Refer to Exhibit 30-6. Explain the stereochemistry of product C.

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
40
Predict the product (including stereochemistry) when the following substance reacts under thermal conditions. 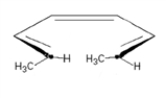


Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
41
Classify the following reaction. 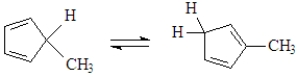
A) electrocyclic reaction
B) cycloaddition
C) sigmatropic rearrangement
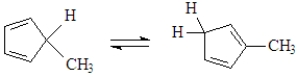
A) electrocyclic reaction
B) cycloaddition
C) sigmatropic rearrangement

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
42
Consider the reaction given below. ![<strong>Consider the reaction given below. Which of the following is correct?</strong> A) [1,5] antarafacial B) [3,3] antarafacial C) [1,5] suprafacial D) [3,5] antarafacial](https://d2lvgg3v3hfg70.cloudfront.net/TB6688/11eab600_07e7_5eb8_9625_59ec0f512d7b_TB6688_00.jpg) Which of the following is correct?
Which of the following is correct?
A) [1,5] antarafacial
B) [3,3] antarafacial
C) [1,5] suprafacial
D) [3,5] antarafacial
![<strong>Consider the reaction given below. Which of the following is correct?</strong> A) [1,5] antarafacial B) [3,3] antarafacial C) [1,5] suprafacial D) [3,5] antarafacial](https://d2lvgg3v3hfg70.cloudfront.net/TB6688/11eab600_07e7_5eb8_9625_59ec0f512d7b_TB6688_00.jpg) Which of the following is correct?
Which of the following is correct?A) [1,5] antarafacial
B) [3,3] antarafacial
C) [1,5] suprafacial
D) [3,5] antarafacial

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
43
Classify the following reaction. 
A) electrocyclic reaction
B) cycloaddition
C) sigmatropic rearrangement

A) electrocyclic reaction
B) cycloaddition
C) sigmatropic rearrangement

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
44
Classify the following reaction. 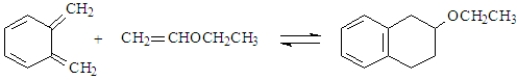
A) electrocyclic reaction
B) cycloaddition
C) sigmatropic rearrangement
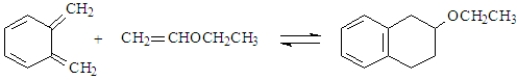
A) electrocyclic reaction
B) cycloaddition
C) sigmatropic rearrangement

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck



