Deck 21: Biomolecules: Carbohydrates
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/49
Play
Full screen (f)
Deck 21: Biomolecules: Carbohydrates
1
Convert the Fischer projections into tetrahedral representations, and assign R or S stereochemistry to each.
Describe the fundamental difference between the following classifications of carbohydrates.
a.
monosaccharide, disaccharide, polysaccharide
b.
simple sugar, complex sugar
c.
ketose, aldose
d.
furanose, pyranose
Describe the fundamental difference between the following classifications of carbohydrates.
a.
monosaccharide, disaccharide, polysaccharide
b.
simple sugar, complex sugar
c.
ketose, aldose
d.
furanose, pyranose
a.the number of monosaccharides present
b.whether or not the carbohydrate can be converted into smaller sugars by hydrolysis
c.
the type of carbonyl group present
d.
the presence of a five or six-membered cyclic hemiacetal
b.whether or not the carbohydrate can be converted into smaller sugars by hydrolysis
c.
the type of carbonyl group present
d.
the presence of a five or six-membered cyclic hemiacetal
2
Refer to the equilibrium below to answer the following question(s). 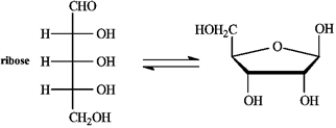
Refer to instructions. Classify ribose by carbonyl type and number of carbons.

Refer to instructions. Classify ribose by carbonyl type and number of carbons.
Ribose is an aldopentose.
3
Refer to the monosaccharides below to answer the following question(s). Classify each sugar by type; for example, glucose is an aldohexose.a. Sorbose 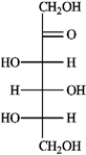 b. Rhamnose
b. Rhamnose 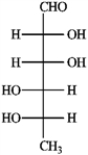 c. Erythrulose
c. Erythrulose 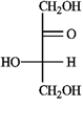 d. Xylulose
d. Xylulose 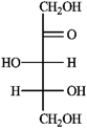
Refer to instructions. Sorbose is ____________________.
 b. Rhamnose
b. Rhamnose  c. Erythrulose
c. Erythrulose  d. Xylulose
d. Xylulose 
Refer to instructions. Sorbose is ____________________.
a ketohexose
4
Convert the Fischer projections into tetrahedral representations, and assign R or S stereochemistry to each.
Convert and assign: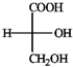
Convert and assign:


Unlock Deck
Unlock for access to all 49 flashcards in this deck.
Unlock Deck
k this deck
5
If the following compound undergoes oxidation in the presence of the appropriate enzyme, draw the structure of the likely product. 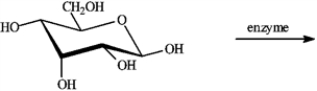


Unlock Deck
Unlock for access to all 49 flashcards in this deck.
Unlock Deck
k this deck
6
Refer to the sugars below to answer the following question(s). Choose the sugar that best fits each description. There is only one correct answer for each question, but sugars may be used more than once. Indicate each answer by the corresponding letter.
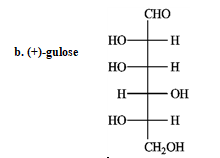
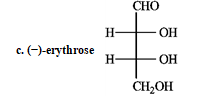
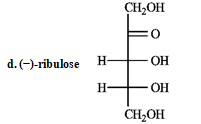
Refer to instructions. _____ a dextrorotary hexose and ______ a levorototary tetrose.
A)a
B)b
C)c
D)d




Refer to instructions. _____ a dextrorotary hexose and ______ a levorototary tetrose.
A)a
B)b
C)c
D)d

Unlock Deck
Unlock for access to all 49 flashcards in this deck.
Unlock Deck
k this deck
7
Refer to the monosaccharides below to answer the following question(s).
a. Sorbose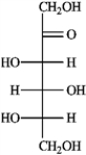 b. Rhamnose
b. Rhamnose 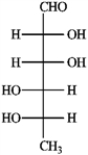 c. Erythrulose
c. Erythrulose 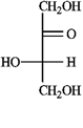 d. Xylulose
d. Xylulose 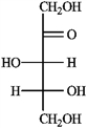
Refer to instructions. Draw both chair conformations of the α-anomer of rhamnose in its pyranose form. Circle the more stable conformation.
a. Sorbose
 b. Rhamnose
b. Rhamnose  c. Erythrulose
c. Erythrulose  d. Xylulose
d. Xylulose 
Refer to instructions. Draw both chair conformations of the α-anomer of rhamnose in its pyranose form. Circle the more stable conformation.

Unlock Deck
Unlock for access to all 49 flashcards in this deck.
Unlock Deck
k this deck
8
Refer to the monosaccharides below to answer the following question(s). Classify each sugar by type; for example, glucose is an aldohexose.a. Sorbose  b. Rhamnose
b. Rhamnose  c. Erythrulose
c. Erythrulose  d. Xylulose
d. Xylulose 
Refer to instructions. Erythrulose is ____________________.
 b. Rhamnose
b. Rhamnose  c. Erythrulose
c. Erythrulose  d. Xylulose
d. Xylulose 
Refer to instructions. Erythrulose is ____________________.

Unlock Deck
Unlock for access to all 49 flashcards in this deck.
Unlock Deck
k this deck
9
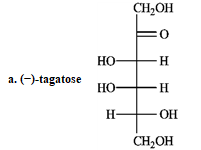
Refer to the sugars below to answer the following question(s). Choose the sugar that best fits each description. There is only one correct answer for each question, but sugars may be used more than once. Indicate each answer by the corresponding letter.




Refer to instructions. _____ oxidizes to an optically inactive aldaric acid.
A)(−)-tagatose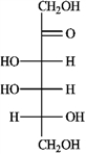
B)(+)-gulose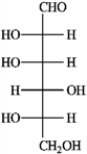
C)(−)-erythrose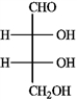
D)(−)-ribulose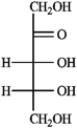




Refer to instructions. _____ oxidizes to an optically inactive aldaric acid.
A)(−)-tagatose

B)(+)-gulose

C)(−)-erythrose

D)(−)-ribulose


Unlock Deck
Unlock for access to all 49 flashcards in this deck.
Unlock Deck
k this deck
10
Refer to the sugars below to answer the following question(s). Choose the sugar that best fits each description. There is only one correct answer for each question, but sugars may be used more than once. Indicate each answer by the corresponding letter.




Complete the equation for the following reaction.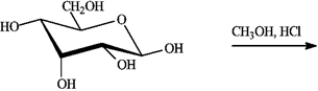




Complete the equation for the following reaction.


Unlock Deck
Unlock for access to all 49 flashcards in this deck.
Unlock Deck
k this deck
11
Refer to the monosaccharides below to answer the following question(s).
a. Sorbose b. Rhamnose
b. Rhamnose  c. Erythrulose
c. Erythrulose  d. Xylulose
d. Xylulose 
Convert the following Fisher projections into tetrahedral representations.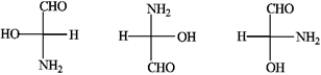
a. Sorbose
 b. Rhamnose
b. Rhamnose  c. Erythrulose
c. Erythrulose  d. Xylulose
d. Xylulose 
Convert the following Fisher projections into tetrahedral representations.


Unlock Deck
Unlock for access to all 49 flashcards in this deck.
Unlock Deck
k this deck
12
Refer to the monosaccharides below to answer the following question(s).
a. Sorbose b. Rhamnose
b. Rhamnose  c. Erythrulose
c. Erythrulose  d. Xylulose
d. Xylulose 
Refer to instructions. Provide the complete name for the α-anomer of rhamnose in its pyranose form.
a. Sorbose
 b. Rhamnose
b. Rhamnose  c. Erythrulose
c. Erythrulose  d. Xylulose
d. Xylulose 
Refer to instructions. Provide the complete name for the α-anomer of rhamnose in its pyranose form.

Unlock Deck
Unlock for access to all 49 flashcards in this deck.
Unlock Deck
k this deck
13
Refer to the sugars below to answer the following question(s). Choose the sugar that best fits each description. There is only one correct answer for each question, but sugars may be used more than once. Indicate each answer by the corresponding letter.




Using the following structure of D-talose as a guide, draw the structure for α-D-talopyranose. Label each ring substituent as axial or equatorial.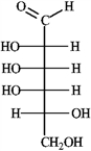




Using the following structure of D-talose as a guide, draw the structure for α-D-talopyranose. Label each ring substituent as axial or equatorial.


Unlock Deck
Unlock for access to all 49 flashcards in this deck.
Unlock Deck
k this deck
14
Refer to the sugars below to answer the following question(s). Choose the sugar that best fits each description. There is only one correct answer for each question, but sugars may be used more than once. Indicate each answer by the corresponding letter.




Draw the structure of the product of the following reaction.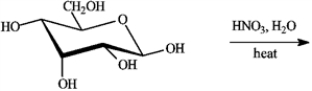




Draw the structure of the product of the following reaction.


Unlock Deck
Unlock for access to all 49 flashcards in this deck.
Unlock Deck
k this deck
15
Refer to the sugars below to answer the following question(s). Choose the sugar that best fits each description. There is only one correct answer for each question, but sugars may be used more than once. Indicate each answer by the corresponding letter.




Refer to instructions. _____ a ketose and _____ an aldose with two chirality centers
A)a
B)b
C)c
D)d




Refer to instructions. _____ a ketose and _____ an aldose with two chirality centers
A)a
B)b
C)c
D)d

Unlock Deck
Unlock for access to all 49 flashcards in this deck.
Unlock Deck
k this deck
16
Convert the Fischer projections into tetrahedral representations, and assign R or S stereochemistry to each.
Convert and assign: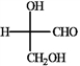
Convert and assign:


Unlock Deck
Unlock for access to all 49 flashcards in this deck.
Unlock Deck
k this deck
17
Refer to the equilibrium below to answer the following question(s). 
Refer to instructions. The correct name for the cyclic structure is____.
A) α-L-ribofuranose
B) β-D-ribofuranose
C) α-L-ribopyranose
D) β-D-ribopyranose

Refer to instructions. The correct name for the cyclic structure is____.
A) α-L-ribofuranose
B) β-D-ribofuranose
C) α-L-ribopyranose
D) β-D-ribopyranose

Unlock Deck
Unlock for access to all 49 flashcards in this deck.
Unlock Deck
k this deck
18
Refer to the monosaccharides below to answer the following question(s).
a. Sorbose b. Rhamnose
b. Rhamnose  c. Erythrulose
c. Erythrulose  d. Xylulose
d. Xylulose 
Refer to instructions. Assign R or S configuration to each chirality center in sorbose.
a. Sorbose
 b. Rhamnose
b. Rhamnose  c. Erythrulose
c. Erythrulose  d. Xylulose
d. Xylulose 
Refer to instructions. Assign R or S configuration to each chirality center in sorbose.

Unlock Deck
Unlock for access to all 49 flashcards in this deck.
Unlock Deck
k this deck
19
Refer to the equilibrium below to answer the following question(s). 
Refer to instructions. Which enantiomer of ribose is drawn, D or L, and is it the α or β anomer?

Refer to instructions. Which enantiomer of ribose is drawn, D or L, and is it the α or β anomer?

Unlock Deck
Unlock for access to all 49 flashcards in this deck.
Unlock Deck
k this deck
20
Refer to the sugars below to answer the following question(s). Choose the sugar that best fits each description. There is only one correct answer for each question, but sugars may be used more than once. Indicate each answer by the corresponding letter.
Refer to instructions. _____ oxidizes to an optically inactive aldaric acid.
A)(-)-tagatose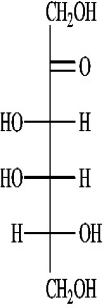
B)(+)-gulose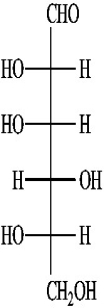
C)(-)-erythrose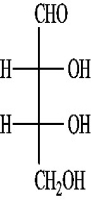
D)(-)-ribulose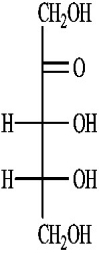
Refer to instructions. _____ oxidizes to an optically inactive aldaric acid.
A)(-)-tagatose

B)(+)-gulose

C)(-)-erythrose

D)(-)-ribulose


Unlock Deck
Unlock for access to all 49 flashcards in this deck.
Unlock Deck
k this deck
21
The monosaccharide shown below is 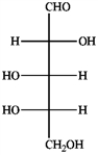
A) an aldohexose
B) an aldopentose
C) an aldotetrose
D) a ketohexose
E) a ketopentose

A) an aldohexose
B) an aldopentose
C) an aldotetrose
D) a ketohexose
E) a ketopentose

Unlock Deck
Unlock for access to all 49 flashcards in this deck.
Unlock Deck
k this deck
22
The specific rotation of the pure α-anomer of a monosaccharide is +169.7 and that of the pure β-anomer is +29.4. A solution of the pure α-anomer produces a specific rotation of +61.4. Based on this data, which of the following is correct?
A) The solution contains more of the α-anomer.
B) The solution is an equimolar mixture of the two anomers.
C) A solution of the pure β-anomer would produce the same specific rotation.
D) The change in specific rotation is due to the irreversible formation of the open-chain monosaccharide.
E) All of these.
A) The solution contains more of the α-anomer.
B) The solution is an equimolar mixture of the two anomers.
C) A solution of the pure β-anomer would produce the same specific rotation.
D) The change in specific rotation is due to the irreversible formation of the open-chain monosaccharide.
E) All of these.

Unlock Deck
Unlock for access to all 49 flashcards in this deck.
Unlock Deck
k this deck
23
What is the correct structure for α-D-glucopyranose?
A)
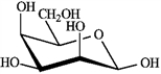
B)
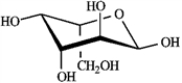
C)
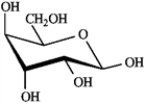
D)
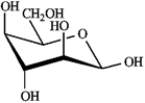
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 49 flashcards in this deck.
Unlock Deck
k this deck
24
Aldohexoses may exist as furanoses or pyranoses. Aldopentoses also may exist as furanoses or pyranoses.
a)Draw Haworth projections for a furanose and a pyranose obtained from D-ribose.
b)Each product can exist in two anomeric forms. What is the structural difference between these forms?
a)Draw Haworth projections for a furanose and a pyranose obtained from D-ribose.
b)Each product can exist in two anomeric forms. What is the structural difference between these forms?

Unlock Deck
Unlock for access to all 49 flashcards in this deck.
Unlock Deck
k this deck
25
Draw structures for the products you would expect to obtain from reaction of β-D-galactopyranose with the listed reagents. Be sure to include all relevant stereochemistry. 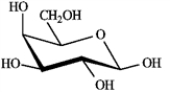 β-D-galactopyranose
β-D-galactopyranose
Draw:
NaBH4 in H2O
 β-D-galactopyranose
β-D-galactopyranoseDraw:
NaBH4 in H2O

Unlock Deck
Unlock for access to all 49 flashcards in this deck.
Unlock Deck
k this deck
26
Draw structures for the products you would expect to obtain from reaction of β-D-galactopyranose with the listed reagents. Be sure to include all relevant stereochemistry.  β-D-galactopyranose
β-D-galactopyranose
Draw:
Br2, H2O
 β-D-galactopyranose
β-D-galactopyranoseDraw:
Br2, H2O

Unlock Deck
Unlock for access to all 49 flashcards in this deck.
Unlock Deck
k this deck
27
Which of the following could not form a pyranose?
A) D-threose
B) D-ribose
C) D-lyxose
D) D-glucose
E) D-galactose
A) D-threose
B) D-ribose
C) D-lyxose
D) D-glucose
E) D-galactose

Unlock Deck
Unlock for access to all 49 flashcards in this deck.
Unlock Deck
k this deck
28
Consider the reaction below to answer the following question(s). 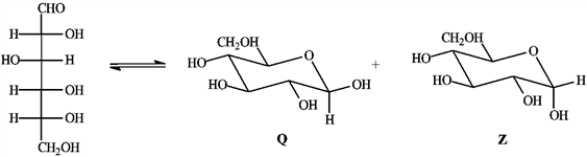
Refer to instructions. Compound Z is:
A) the D-anomer.
B) the α-anomer.
C) the β-anomer.
D) the L-anomer.

Refer to instructions. Compound Z is:
A) the D-anomer.
B) the α-anomer.
C) the β-anomer.
D) the L-anomer.

Unlock Deck
Unlock for access to all 49 flashcards in this deck.
Unlock Deck
k this deck
29
Consider the reaction below to answer the following question(s). 
Refer to instructions. Which anomer is the LEAST stable?
Q or Z

Refer to instructions. Which anomer is the LEAST stable?
Q or Z

Unlock Deck
Unlock for access to all 49 flashcards in this deck.
Unlock Deck
k this deck
30
Consider the reaction below to answer the following question(s). 
Refer to instructions. Q and Z are cyclic examples of:
A) acetals
B) hemiacetals
C) alditols
D) hemialditols

Refer to instructions. Q and Z are cyclic examples of:
A) acetals
B) hemiacetals
C) alditols
D) hemialditols

Unlock Deck
Unlock for access to all 49 flashcards in this deck.
Unlock Deck
k this deck
31
Refer to the sugars below to answer the following question(s). Choose the sugar that best fits each description. There is only one correct answer for each question, but sugars may be used more than once. Indicate each answer by the corresponding letter.
Draw the Fisher projection of L-mannose.
Draw the Fisher projection of L-mannose.

Unlock Deck
Unlock for access to all 49 flashcards in this deck.
Unlock Deck
k this deck
32
Draw an aldopentose with S,S,R stereochemistries at its carbons 2 through 4.

Unlock Deck
Unlock for access to all 49 flashcards in this deck.
Unlock Deck
k this deck
33
Refer to the sugars below to answer the following question(s). Choose the sugar that best fits each description. There is only one correct answer for each question, but sugars may be used more than once. Indicate each answer by the corresponding letter.
Refer to instructions. _____ a ketose and _____ an aldose with two chirality centers
A)(-)-tagatose
B)(+)-gulose
C)(-)-erythrose
D)(-)-ribulose
Refer to instructions. _____ a ketose and _____ an aldose with two chirality centers
A)(-)-tagatose

B)(+)-gulose

C)(-)-erythrose

D)(-)-ribulose


Unlock Deck
Unlock for access to all 49 flashcards in this deck.
Unlock Deck
k this deck
34
Refer to D-iodose below to answer the following question(s). 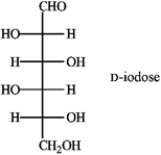
Refer to instructions. The Fischer projection for D-iodose (above) corresponds to which pyranose below?
A)
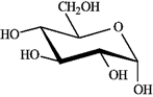
B)
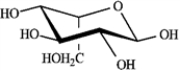
C)
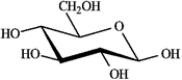
D)
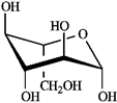

Refer to instructions. The Fischer projection for D-iodose (above) corresponds to which pyranose below?
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 49 flashcards in this deck.
Unlock Deck
k this deck
35
How many D-ketotetroses could exist?
A) none
B) one
C) two
D) four
E) eight
A) none
B) one
C) two
D) four
E) eight

Unlock Deck
Unlock for access to all 49 flashcards in this deck.
Unlock Deck
k this deck
36
Refer to the carbohydrate below to answer the following questions. 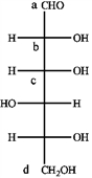 a)Which carbon in the carbohydrate is the anomeric carbon?
a)Which carbon in the carbohydrate is the anomeric carbon?
b)What would be observed on the addition of Tollen's reagent to a solution of this sugar?
 a)Which carbon in the carbohydrate is the anomeric carbon?
a)Which carbon in the carbohydrate is the anomeric carbon?b)What would be observed on the addition of Tollen's reagent to a solution of this sugar?

Unlock Deck
Unlock for access to all 49 flashcards in this deck.
Unlock Deck
k this deck
37
Draw structures for the products you would expect to obtain from reaction of β-D-galactopyranose with the listed reagents. Be sure to include all relevant stereochemistry.  β-D-galactopyranose
β-D-galactopyranose
Draw:
Ch3I, Ag2O
 β-D-galactopyranose
β-D-galactopyranoseDraw:
Ch3I, Ag2O

Unlock Deck
Unlock for access to all 49 flashcards in this deck.
Unlock Deck
k this deck
38
Refer to the sugars below to answer the following question(s). Choose the sugar that best fits each description. There is only one correct answer for each question, but sugars may be used more than once. Indicate each answer by the corresponding letter.
Refer to instructions. _____ a dextrorotary hexose and ______ a levorototary tetrose.
A)(-)-tagatose
B)(+)-gulose
C)(-)-erythrose
D)(-)-ribulose
Refer to instructions. _____ a dextrorotary hexose and ______ a levorototary tetrose.
A)(-)-tagatose

B)(+)-gulose

C)(-)-erythrose

D)(-)-ribulose


Unlock Deck
Unlock for access to all 49 flashcards in this deck.
Unlock Deck
k this deck
39
Refer to the carbohydrate below to answer the following questions. 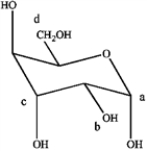 a)Which carbon in the carbohydrate is the anomeric carbon?
a)Which carbon in the carbohydrate is the anomeric carbon?
b)Does this represent an α or β-anomoer?
 a)Which carbon in the carbohydrate is the anomeric carbon?
a)Which carbon in the carbohydrate is the anomeric carbon?b)Does this represent an α or β-anomoer?

Unlock Deck
Unlock for access to all 49 flashcards in this deck.
Unlock Deck
k this deck
40
Consider the reaction below to answer the following question(s). 
Refer to instructions. Place a triangle around the anomeric carbon in compound Q.

Refer to instructions. Place a triangle around the anomeric carbon in compound Q.

Unlock Deck
Unlock for access to all 49 flashcards in this deck.
Unlock Deck
k this deck
41
Choose a structure from the list provided below that best fits the description given. There is only one correct answer for each question.
A non-reducing disaccharide
A)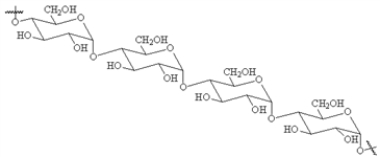
B)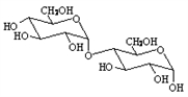
C)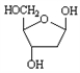
D)
E)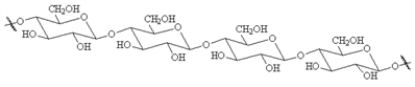
F)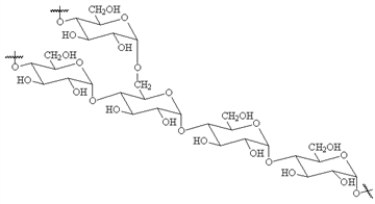
A non-reducing disaccharide
A)

B)

C)

D)

E)

F)


Unlock Deck
Unlock for access to all 49 flashcards in this deck.
Unlock Deck
k this deck
42
Which of the following is a disaccharide?
A) glucose
B) fructose
C) sucrose
D) N-acetylgalactosamine
E) both c and d
A) glucose
B) fructose
C) sucrose
D) N-acetylgalactosamine
E) both c and d

Unlock Deck
Unlock for access to all 49 flashcards in this deck.
Unlock Deck
k this deck
43
What is the relationship between D-erythrose and D-threose? 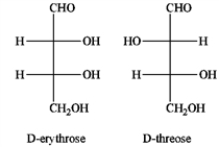
A) they are constitutional isomers
B) they are enantiomers
C) they are diastereomers
D) they are tautomers

A) they are constitutional isomers
B) they are enantiomers
C) they are diastereomers
D) they are tautomers

Unlock Deck
Unlock for access to all 49 flashcards in this deck.
Unlock Deck
k this deck
44
Choose a structure from the list provided below that best fits the description given. There is only one correct answer for each question.
A reducing monosaccharide
A)
B)
C)
D)
E)
F)
A reducing monosaccharide
A)

B)

C)

D)

E)

F)


Unlock Deck
Unlock for access to all 49 flashcards in this deck.
Unlock Deck
k this deck
45
Which of the following has a D-configuration? 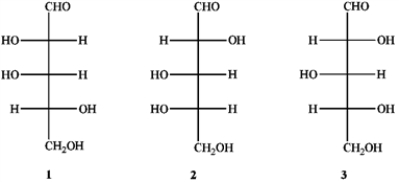
A) only 1 and 2
B) only 1 and 3
C) only 2 and 3
D) only 1, 2 and 3

A) only 1 and 2
B) only 1 and 3
C) only 2 and 3
D) only 1, 2 and 3

Unlock Deck
Unlock for access to all 49 flashcards in this deck.
Unlock Deck
k this deck
46
Choose a structure from the list provided below that best fits the description given. There is only one correct answer for each question.
A 1,4' β-glycoside
A)
B)
C)
D)
E)
F)
A 1,4' β-glycoside
A)

B)

C)

D)

E)

F)


Unlock Deck
Unlock for access to all 49 flashcards in this deck.
Unlock Deck
k this deck
47
Treatment with dilute NaOH can convert
A) an aldose into a ketose
B) an aldose into a carboxylic acid
C) an aldose into its acetate ester
D) a glycoside into a monosaccharide plus an alcohol
E) a monosaccharide plus an alcohol into a glycoside
A) an aldose into a ketose
B) an aldose into a carboxylic acid
C) an aldose into its acetate ester
D) a glycoside into a monosaccharide plus an alcohol
E) a monosaccharide plus an alcohol into a glycoside

Unlock Deck
Unlock for access to all 49 flashcards in this deck.
Unlock Deck
k this deck
48
Which reagent would be best suited for the transformation shown? 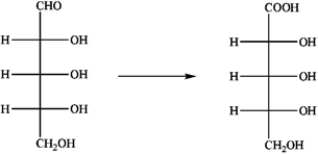
A) alkaline Cu2+ in H2O
B) Ag+ in H2O/NH3
C) H2, with Ni catalyst
D) NaNO3 at 0°C
E) NaBH4 in H2O

A) alkaline Cu2+ in H2O
B) Ag+ in H2O/NH3
C) H2, with Ni catalyst
D) NaNO3 at 0°C
E) NaBH4 in H2O

Unlock Deck
Unlock for access to all 49 flashcards in this deck.
Unlock Deck
k this deck
49
The brick-red color observed when Benedict's test is applied to D-mannose is due to
A) the reduced form of the Cu2+ reagent
B) the reduced form of the D-mannose
C) the oxidized form of the Cu2+ reagent
D) the oxidized form of the D-mannose
E) products in the mixture other than those listed
A) the reduced form of the Cu2+ reagent
B) the reduced form of the D-mannose
C) the oxidized form of the Cu2+ reagent
D) the oxidized form of the D-mannose
E) products in the mixture other than those listed

Unlock Deck
Unlock for access to all 49 flashcards in this deck.
Unlock Deck
k this deck



