Deck 3: Organic Compounds: Alkanes and Their Stereochemistry
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/32
Play
Full screen (f)
Deck 3: Organic Compounds: Alkanes and Their Stereochemistry
1
Which hydrogen atom(s) in the following compound is (are) classified as tertiary? 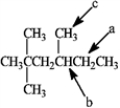
A) a
B) b
C) c
D) both a and c
E) There are no tertiary hydrogen atoms.

A) a
B) b
C) c
D) both a and c
E) There are no tertiary hydrogen atoms.
b
2
Circle and name each functional group in the following structure. 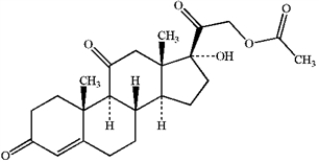 cortisone acetate (active ingredient in steroid skin cream)
cortisone acetate (active ingredient in steroid skin cream)
 cortisone acetate (active ingredient in steroid skin cream)
cortisone acetate (active ingredient in steroid skin cream)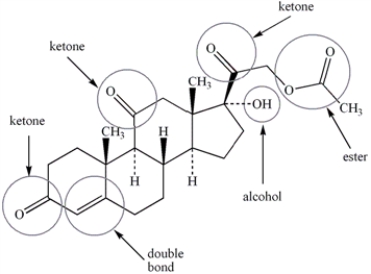
3
Draw the structure of a five carbon ketone containing one tertiary carbon atom.
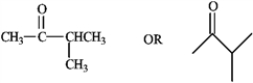
4
Experiments have shown that for 1,2-dichloroethane, ClCH2CH2Cl, in tetrachloromethane solution at 25° C, 70% of the molecules are in the anti and 30% are in the gauche conformation. Use this information to answer the following question(s).
Refer to instructions. Draw a Newman projection of the highest energy eclipsed conformation of 1,2-dichloroethane.
Refer to instructions. Draw a Newman projection of the highest energy eclipsed conformation of 1,2-dichloroethane.

Unlock Deck
Unlock for access to all 32 flashcards in this deck.
Unlock Deck
k this deck
5
Provide the line bond structure for 4-(2,2-dibromoethyl)-3,5-dichloroheptane.

Unlock Deck
Unlock for access to all 32 flashcards in this deck.
Unlock Deck
k this deck
6
Match the Newman projection for the conformation of 2-methylbutane to the indicated position on the potential energy diagram. 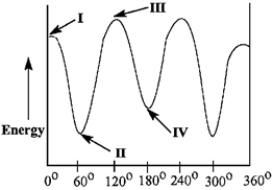 A
A 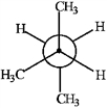 B
B 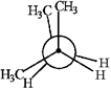 C
C 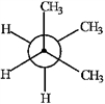 D
D 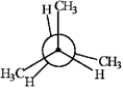
Refer to instructions. Conformations A and D are represented by the Roman numerals _____ and _____ in the diagram, respectively.
 A
A  B
B  C
C  D
D 
Refer to instructions. Conformations A and D are represented by the Roman numerals _____ and _____ in the diagram, respectively.

Unlock Deck
Unlock for access to all 32 flashcards in this deck.
Unlock Deck
k this deck
7
One of the functional group classifications is characterized by the presence of an sp2 hybridized carbon atom. This functional group could be:
A) alkyl halide
B) sulfide
C) alcohol
D) aldehyde
E) alkyne
A) alkyl halide
B) sulfide
C) alcohol
D) aldehyde
E) alkyne

Unlock Deck
Unlock for access to all 32 flashcards in this deck.
Unlock Deck
k this deck
8
The carbon atoms within the functional group of the following classifications are: alkene ester carboxylic acid amide
A) sp
B) sp2
C) sp3
D) sp2 and sp3
A) sp
B) sp2
C) sp3
D) sp2 and sp3

Unlock Deck
Unlock for access to all 32 flashcards in this deck.
Unlock Deck
k this deck
9
Which of the following functional group classifications do not contain oxygen?
A) ether
B) thiol
C) aldehyde
D) ester
E) amide
A) ether
B) thiol
C) aldehyde
D) ester
E) amide

Unlock Deck
Unlock for access to all 32 flashcards in this deck.
Unlock Deck
k this deck
10
Provide a proper IUPAC name for the compound given below. 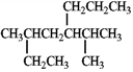


Unlock Deck
Unlock for access to all 32 flashcards in this deck.
Unlock Deck
k this deck
11
Match the Newman projection for the conformation of 2-methylbutane to the indicated position on the potential energy diagram.  A
A  B
B  C
C  D
D 
Refer to instructions. Conformations B and C are represented by the Roman numerals _____ and _____ in the diagram, respectively.
 A
A  B
B  C
C  D
D 
Refer to instructions. Conformations B and C are represented by the Roman numerals _____ and _____ in the diagram, respectively.

Unlock Deck
Unlock for access to all 32 flashcards in this deck.
Unlock Deck
k this deck
12
Among the following structures, how many different compounds are represented? 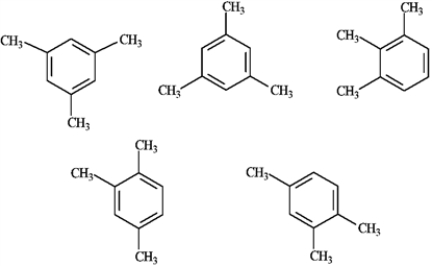
A) 5
B) 4
C) 3
D) 2
E) 1

A) 5
B) 4
C) 3
D) 2
E) 1

Unlock Deck
Unlock for access to all 32 flashcards in this deck.
Unlock Deck
k this deck
13
If ethane reacts with a large excess of chlorine over a long period of time, the most likely major product of the reaction would be:
A) CCl3CCl3
B) CH3CH3
C) CH2ClCH2Cl
D) CH2ClCH3
E) Ethane does not react with the halogens.
A) CCl3CCl3
B) CH3CH3
C) CH2ClCH2Cl
D) CH2ClCH3
E) Ethane does not react with the halogens.

Unlock Deck
Unlock for access to all 32 flashcards in this deck.
Unlock Deck
k this deck
14
To which functional group classification does the following molecule belong? 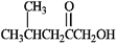
A) ester
B) ketone
C) alcohol
D) carboxylic acid
E) both b and c
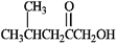
A) ester
B) ketone
C) alcohol
D) carboxylic acid
E) both b and c

Unlock Deck
Unlock for access to all 32 flashcards in this deck.
Unlock Deck
k this deck
15
Experiments have shown that for 1,2-dichloroethane, ClCH2CH2Cl, in tetrachloromethane solution at 25° C, 70% of the molecules are in the anti and 30% are in the gauche conformation. Use this information to answer the following question(s).
Refer to instructions. Draw a Newman projection of the anti conformation of 1,2-dichloroethane.
Refer to instructions. Draw a Newman projection of the anti conformation of 1,2-dichloroethane.

Unlock Deck
Unlock for access to all 32 flashcards in this deck.
Unlock Deck
k this deck
16
Experiments have shown that for 1,2-dichloroethane, ClCH2CH2Cl, in tetrachloromethane solution at 25° C, 70% of the molecules are in the anti and 30% are in the gauche conformation. Use this information to answer the following question(s).
Refer to instructions. Draw a Newman projection of the gauche conformation of 1,2-dichloroethane.
Refer to instructions. Draw a Newman projection of the gauche conformation of 1,2-dichloroethane.

Unlock Deck
Unlock for access to all 32 flashcards in this deck.
Unlock Deck
k this deck
17
4-ethyl-3,3,4-trimethylheptane could be classified as:
A) an alkane
B) saturated
C) aliphatic
D) a paraffin
E) all of these
A) an alkane
B) saturated
C) aliphatic
D) a paraffin
E) all of these

Unlock Deck
Unlock for access to all 32 flashcards in this deck.
Unlock Deck
k this deck
18
4-ethyl-3,3,4-trimethylheptane contains:
A) two quaternary carbon atoms
B) two tertiary carbon atoms
C) four secondary carbon atoms
D) a and c
E) all of these
A) two quaternary carbon atoms
B) two tertiary carbon atoms
C) four secondary carbon atoms
D) a and c
E) all of these

Unlock Deck
Unlock for access to all 32 flashcards in this deck.
Unlock Deck
k this deck
19
Label the following pair of compounds as: 
A) identical
B) constitutional isomers
C) stereoisomers
D) unrelated

A) identical
B) constitutional isomers
C) stereoisomers
D) unrelated

Unlock Deck
Unlock for access to all 32 flashcards in this deck.
Unlock Deck
k this deck
20
Provide a line bond structure for 5-tert-butyl-2,3-dimethyloctane.

Unlock Deck
Unlock for access to all 32 flashcards in this deck.
Unlock Deck
k this deck
21
The most stable conformation of an alkane occurs when
A) carbon−carbon bonds are staggered and bulky groups are gauche.
B) carbon−carbon bonds are staggered and bulky groups are anti.
C) carbon−carbon bonds are eclipsed and bulky groups are gauche.
D) carbon−carbon bonds are eclipsed and bulky groups are anti.
A) carbon−carbon bonds are staggered and bulky groups are gauche.
B) carbon−carbon bonds are staggered and bulky groups are anti.
C) carbon−carbon bonds are eclipsed and bulky groups are gauche.
D) carbon−carbon bonds are eclipsed and bulky groups are anti.

Unlock Deck
Unlock for access to all 32 flashcards in this deck.
Unlock Deck
k this deck
22
Which of the following Newman projections represents the most stable conformation of 2,3-dimethylbutane?
A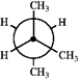 B
B 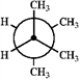 C
C 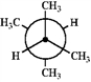 D
D 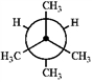
A) A
B) B
C) C
D) D
A
 B
B  C
C  D
D 
A) A
B) B
C) C
D) D

Unlock Deck
Unlock for access to all 32 flashcards in this deck.
Unlock Deck
k this deck
23
Name these groups (left to right). 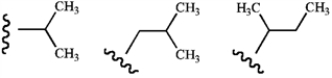 where
where  represents the parent chain.
represents the parent chain.
A) sec-propyl, sec-butyl, isobutyl
B) isopropyl, isobutyl, sec-butyl
C) sec-propyl, tert-butyl, isobutyl
D) isopropyl, tert-butyl, isobutyl
E) isopropyl, tert-butyl, sec-butyl
 where
where  represents the parent chain.
represents the parent chain.A) sec-propyl, sec-butyl, isobutyl
B) isopropyl, isobutyl, sec-butyl
C) sec-propyl, tert-butyl, isobutyl
D) isopropyl, tert-butyl, isobutyl
E) isopropyl, tert-butyl, sec-butyl

Unlock Deck
Unlock for access to all 32 flashcards in this deck.
Unlock Deck
k this deck
24
Which of the following alkanes is the most likely to be a liquid at room temperature?
A) propane
B) butane
C) pentane
D) hexane
A) propane
B) butane
C) pentane
D) hexane

Unlock Deck
Unlock for access to all 32 flashcards in this deck.
Unlock Deck
k this deck
25
Choose a correct IUPAC name for: 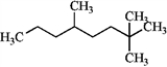
A) 3-methyl-l-tert-butylhexane
B) 5-methyloctane
C) 2,5-dimethylheptane
D) 4,7,7-trimethyloctane
E) none of these

A) 3-methyl-l-tert-butylhexane
B) 5-methyloctane
C) 2,5-dimethylheptane
D) 4,7,7-trimethyloctane
E) none of these

Unlock Deck
Unlock for access to all 32 flashcards in this deck.
Unlock Deck
k this deck
26
Consider the conformations of 2-methylbutane shown below to answer the following question(s).
A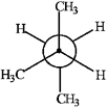 B
B 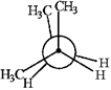 C
C 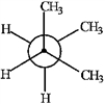 D
D 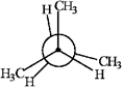 a.A
a.A
b.B
c.C
d.D
Which of the structures represents the most stable conformation of 2-methylbutane?
A
 B
B  C
C  D
D  a.A
a.Ab.B
c.C
d.D
Which of the structures represents the most stable conformation of 2-methylbutane?

Unlock Deck
Unlock for access to all 32 flashcards in this deck.
Unlock Deck
k this deck
27
What is the IUPAC name of the following compound? 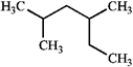
A) 2-ethyl-4-methylpentane
B) 2,4-dimethylhexane
C) 3,5-dimethylhexane
D) 1,1,3-trimethylpentane

A) 2-ethyl-4-methylpentane
B) 2,4-dimethylhexane
C) 3,5-dimethylhexane
D) 1,1,3-trimethylpentane

Unlock Deck
Unlock for access to all 32 flashcards in this deck.
Unlock Deck
k this deck
28
How many constitutional isomers are there with the molecular formula C6H14?
A) 3
B) 4
C) 5
D) 8
A) 3
B) 4
C) 5
D) 8

Unlock Deck
Unlock for access to all 32 flashcards in this deck.
Unlock Deck
k this deck
29
Consider the conformations of 2-methylbutane shown below to answer the following question(s).
A B
B  C
C  D
D  a.A
a.A
b.B
c.C
d.D
Which of the structures has two gauche interactions?
A
 B
B  C
C  D
D  a.A
a.Ab.B
c.C
d.D
Which of the structures has two gauche interactions?

Unlock Deck
Unlock for access to all 32 flashcards in this deck.
Unlock Deck
k this deck
30
Pick the set of terms that correctly classifies the compound. 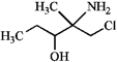
A) tertiary amine, primary alkyl chloride, secondary alcohol
B) primary amine, primary alkyl chloride, secondary alcohol
C) primary amine, primary alkyl chloride, tertiary alcohol
D) secondary amine, primary alkyl chloride, secondary alcohol
E) secondary amine, secondary alkyl chloride, secondary alcohol

A) tertiary amine, primary alkyl chloride, secondary alcohol
B) primary amine, primary alkyl chloride, secondary alcohol
C) primary amine, primary alkyl chloride, tertiary alcohol
D) secondary amine, primary alkyl chloride, secondary alcohol
E) secondary amine, secondary alkyl chloride, secondary alcohol

Unlock Deck
Unlock for access to all 32 flashcards in this deck.
Unlock Deck
k this deck
31
Designate each carbon as primary, secondary, tertiary, or quaternary. 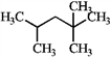


Unlock Deck
Unlock for access to all 32 flashcards in this deck.
Unlock Deck
k this deck
32
Which of the following Newman projections does not represent 2-methylhexane?
A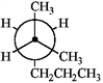 B
B 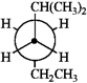 C
C 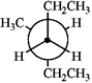 D
D 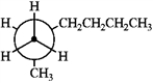
A) A
B) B
C) C
D) D
A
 B
B  C
C  D
D 
A) A
B) B
C) C
D) D

Unlock Deck
Unlock for access to all 32 flashcards in this deck.
Unlock Deck
k this deck



