Deck 20: Carboxylic Acids and Nitriles
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/32
Play
Full screen (f)
Deck 20: Carboxylic Acids and Nitriles
1
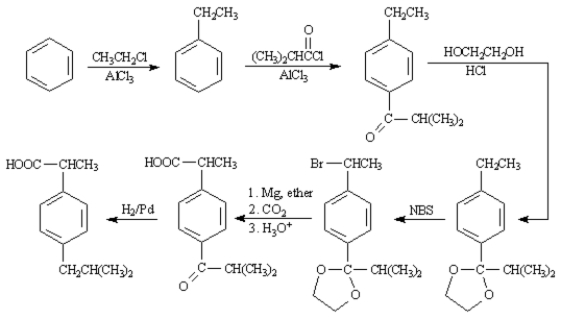
Propose a synthesis of the anti-inflammatory drug Ibuprofen from benzene.Show all reagents and all intermediate structures.Assume that ortho and para isomers can be separated. 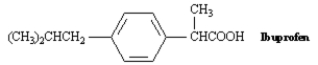

2
Name:
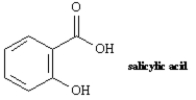

2-hydroxybenzoic acid
3
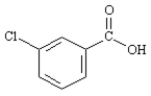
Draw: m-chlorobenzoic acid
4
Draw: 2-propenenitrile

Unlock Deck
Unlock for access to all 32 flashcards in this deck.
Unlock Deck
k this deck
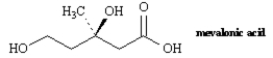
5
Name:


Unlock Deck
Unlock for access to all 32 flashcards in this deck.
Unlock Deck
k this deck
6
Name: 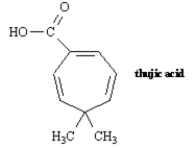


Unlock Deck
Unlock for access to all 32 flashcards in this deck.
Unlock Deck
k this deck
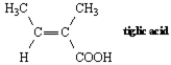
7
Name:


Unlock Deck
Unlock for access to all 32 flashcards in this deck.
Unlock Deck
k this deck
8
Even though the para position is one carbon farther from the carboxy group than the meta position,p-cyanobenzoic acid is more acidic than m-cyanobenzoic acid.Explain the differences in acidity between p-cyanobenzoic acid and m-cyanobenzoic acid.

Unlock Deck
Unlock for access to all 32 flashcards in this deck.
Unlock Deck
k this deck
9
What is the correct name for the following structure? 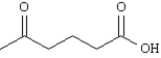
A)2-oxohexanoic acid
B)5-oxohexanoic acid
C)methyl butroxoketone
D)4-ketopentanoic acid

A)2-oxohexanoic acid
B)5-oxohexanoic acid
C)methyl butroxoketone
D)4-ketopentanoic acid

Unlock Deck
Unlock for access to all 32 flashcards in this deck.
Unlock Deck
k this deck
10
Name the following substance.Atoms other than carbon and hydrogen are labeled. 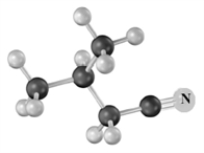


Unlock Deck
Unlock for access to all 32 flashcards in this deck.
Unlock Deck
k this deck
11
Exhibit 20-2
Consider the data below to answer the following question(s).
When CO2 is bubbled through an ether solution of benzylmagnesium bromide,and the resulting mixture is acidified,phenylacetic acid is produced.Any unreacted benzylmagnesium bromide is converted to toluene in the acidification step.
Refer to Exhibit 20-2.Write the complete reaction sequence for the process described above.
Consider the data below to answer the following question(s).
When CO2 is bubbled through an ether solution of benzylmagnesium bromide,and the resulting mixture is acidified,phenylacetic acid is produced.Any unreacted benzylmagnesium bromide is converted to toluene in the acidification step.
Refer to Exhibit 20-2.Write the complete reaction sequence for the process described above.

Unlock Deck
Unlock for access to all 32 flashcards in this deck.
Unlock Deck
k this deck
12
Explain the differences in acidity between p-methoxybenzoic acid and m-methoxybenzoic acid.

Unlock Deck
Unlock for access to all 32 flashcards in this deck.
Unlock Deck
k this deck
13
Exhibit 20-2
Consider the data below to answer the following question(s).
When CO2 is bubbled through an ether solution of benzylmagnesium bromide,and the resulting mixture is acidified,phenylacetic acid is produced.Any unreacted benzylmagnesium bromide is converted to toluene in the acidification step.
Refer to Exhibit 20-2.How could you separate phenylacetic acid from toluene?
Consider the data below to answer the following question(s).
When CO2 is bubbled through an ether solution of benzylmagnesium bromide,and the resulting mixture is acidified,phenylacetic acid is produced.Any unreacted benzylmagnesium bromide is converted to toluene in the acidification step.
Refer to Exhibit 20-2.How could you separate phenylacetic acid from toluene?

Unlock Deck
Unlock for access to all 32 flashcards in this deck.
Unlock Deck
k this deck
14
Name: 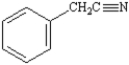


Unlock Deck
Unlock for access to all 32 flashcards in this deck.
Unlock Deck
k this deck
15
Name: 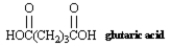

Unlock Deck
Unlock for access to all 32 flashcards in this deck.
Unlock Deck
k this deck
16
Draw: 2-propylpentanoic acid

Unlock Deck
Unlock for access to all 32 flashcards in this deck.
Unlock Deck
k this deck
17
Draw: cis-1,3-cyclopentanedicarboxylic acid

Unlock Deck
Unlock for access to all 32 flashcards in this deck.
Unlock Deck
k this deck
18
Exhibit 20-2
Consider the data below to answer the following question(s).
When CO2 is bubbled through an ether solution of benzylmagnesium bromide,and the resulting mixture is acidified,phenylacetic acid is produced.Any unreacted benzylmagnesium bromide is converted to toluene in the acidification step.
Refer to Exhibit 20-2.This reaction is described as a _____ process.
A)carbonylation
B)carboxylation
C)carbaniolation
D)cationation
Consider the data below to answer the following question(s).
When CO2 is bubbled through an ether solution of benzylmagnesium bromide,and the resulting mixture is acidified,phenylacetic acid is produced.Any unreacted benzylmagnesium bromide is converted to toluene in the acidification step.
Refer to Exhibit 20-2.This reaction is described as a _____ process.
A)carbonylation
B)carboxylation
C)carbaniolation
D)cationation

Unlock Deck
Unlock for access to all 32 flashcards in this deck.
Unlock Deck
k this deck
19
Name the following substance.Atoms other than carbon and hydrogen are labeled. 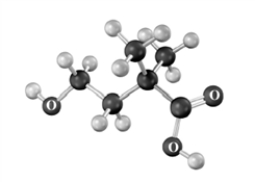


Unlock Deck
Unlock for access to all 32 flashcards in this deck.
Unlock Deck
k this deck
20
Draw: cyanoacetic acid

Unlock Deck
Unlock for access to all 32 flashcards in this deck.
Unlock Deck
k this deck
21
In order to produce a carboxylic acid as a product of the Grignard reaction,the Grignard reagent reacts with:
A)CO2
B)a methyl ketone
C)a methyl ester
D)an aldehyde
E)either or CO2 a methyl ketone
A)CO2
B)a methyl ketone
C)a methyl ester
D)an aldehyde
E)either or CO2 a methyl ketone

Unlock Deck
Unlock for access to all 32 flashcards in this deck.
Unlock Deck
k this deck
22
Which of the following is not an acyl derivative of a carboxylic acid?
A)nitrile
B)ester
C)amide
D)acid chloride
A)nitrile
B)ester
C)amide
D)acid chloride

Unlock Deck
Unlock for access to all 32 flashcards in this deck.
Unlock Deck
k this deck
23
Draw the mechanism of the basic hydrolysis of methyl nitrile to yield methyl amide,which is then hydrolyzed to yield an acetate ion.

Unlock Deck
Unlock for access to all 32 flashcards in this deck.
Unlock Deck
k this deck
24
In a solution of acetic acid ( 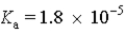 ) at physiological pH of 7.3,it is most accurate to represent this substance as:
) at physiological pH of 7.3,it is most accurate to represent this substance as:
A)CH3CO2H
B)CH3CO2-
C)CH3CO2H and CH3CO2-
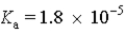 ) at physiological pH of 7.3,it is most accurate to represent this substance as:
) at physiological pH of 7.3,it is most accurate to represent this substance as:A)CH3CO2H
B)CH3CO2-
C)CH3CO2H and CH3CO2-

Unlock Deck
Unlock for access to all 32 flashcards in this deck.
Unlock Deck
k this deck
25
Draw the mechanism for the preparation of nitriles by dehydration of a primary amide, e.g. ,propanamide,using thionyl chloride.

Unlock Deck
Unlock for access to all 32 flashcards in this deck.
Unlock Deck
k this deck
26
A solution of glycolic acid is prepared such that the glycolic acid is 69% dissociated.Calculate the pH of this solution.( 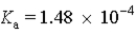 )
)
A)3.83
B)6.06
C)4.18
D)3.48
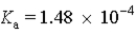 )
)A)3.83
B)6.06
C)4.18
D)3.48

Unlock Deck
Unlock for access to all 32 flashcards in this deck.
Unlock Deck
k this deck
27
Which labeled peaks would disappear if D2O were added to the sample? 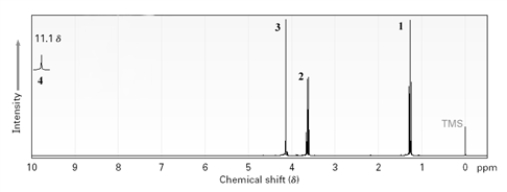
A)1
B)2
C)3
D)4
E)All peaks would disappear.

A)1
B)2
C)3
D)4
E)All peaks would disappear.

Unlock Deck
Unlock for access to all 32 flashcards in this deck.
Unlock Deck
k this deck
28
A sample is known to contain either hexanoic acid or 3-methylpentanoic acid.Which of the following spectra would not distinguish between the two isomers?
A)(1H NMR)
B)(13C NMR)
C)DEPT-90
D)DEPT-135
E)All would distinguish between the two isomers.
A)(1H NMR)
B)(13C NMR)
C)DEPT-90
D)DEPT-135
E)All would distinguish between the two isomers.

Unlock Deck
Unlock for access to all 32 flashcards in this deck.
Unlock Deck
k this deck
29
Draw the mechanism of the conversion of isopropyl nitrile to an amine.

Unlock Deck
Unlock for access to all 32 flashcards in this deck.
Unlock Deck
k this deck
30
Draw the structure of the reactant needed to form the following upon treatment with SOCl2 at a moderately elevated temperature.Atoms other than carbon and hydrogen are labeled. 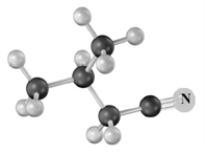


Unlock Deck
Unlock for access to all 32 flashcards in this deck.
Unlock Deck
k this deck
31
Which of the following is not a common feature of both carboxylic acids and nitriles?
A)carbon atom with three bonds to an electronegative atom
B)presence of π bonds
C)can act as electrophiles
D)occur frequently in living organisms
E)All characterize both functional group classes.
A)carbon atom with three bonds to an electronegative atom
B)presence of π bonds
C)can act as electrophiles
D)occur frequently in living organisms
E)All characterize both functional group classes.

Unlock Deck
Unlock for access to all 32 flashcards in this deck.
Unlock Deck
k this deck
32
Draw the structure of the product formed when the following is treated with 1) NaOH (aq) and 2) H3O+.Explain,if necessary how the product would be affected if the treatment with acid were omitted. 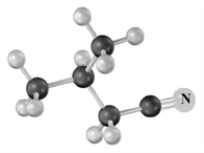


Unlock Deck
Unlock for access to all 32 flashcards in this deck.
Unlock Deck
k this deck



