Deck 17: Alcohols and Phenols
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/44
Play
Full screen (f)
Deck 17: Alcohols and Phenols
1
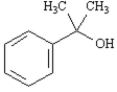
Draw: 2-phenyl-2-propanol
2
Exhibit 17-2
Refer to the data below to answer the following question(s).
pKas of Some Phenols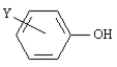 Y
Y
pKa
−H
9.89
m-NO2
8.28
p-NO2
7.17
m-OCH3
9.65
p-OCH3
10.21
Refer to Exhibit 17-2.The weakest acid in the table is:
Refer to the data below to answer the following question(s).
pKas of Some Phenols
 Y
YpKa
−H
9.89
m-NO2
8.28
p-NO2
7.17
m-OCH3
9.65
p-OCH3
10.21
Refer to Exhibit 17-2.The weakest acid in the table is:
p-methoxyphenol
3
Refer to Exhibit 17-3.On the templates provided below,draw both conformations of the alcohol product.Circle the least stable conformation. 


4
Exhibit 17-4
To answer the following question(s),consider the reaction below: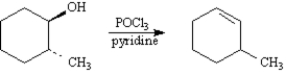
Refer to Exhibit 17-4.On the structures provided below,draw arrows which account for the complete stepwise mechanism for this reaction.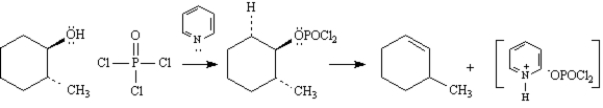
To answer the following question(s),consider the reaction below:

Refer to Exhibit 17-4.On the structures provided below,draw arrows which account for the complete stepwise mechanism for this reaction.


Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
5
Explain why nonafluoro-2-methyl-2-propoxide is a much weaker base than tert-butoxide. 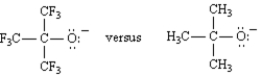
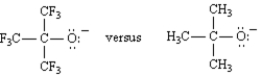

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
6
Refer to Exhibit 17-3.Provide the IUPAC name for the product alcohol.

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
7
H2O2,−OH

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
8
Exhibit 17-4
To answer the following question(s),consider the reaction below:
Refer to Exhibit 17-4.The dehydration of secondary and tertiary alcohols by reaction with POCl3 in pyridine is an example of:
A)an E1 process
B)an SN1 process
C)an E2 process
D)an SN2 process
To answer the following question(s),consider the reaction below:

Refer to Exhibit 17-4.The dehydration of secondary and tertiary alcohols by reaction with POCl3 in pyridine is an example of:
A)an E1 process
B)an SN1 process
C)an E2 process
D)an SN2 process

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
9
Refer to Exhibit 17-3.The alcohol product is classified as a:
A)1° alcohol
B)2° alcohol
C)3° alcohol
D)4° alcohol
A)1° alcohol
B)2° alcohol
C)3° alcohol
D)4° alcohol

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
10
Draw: cis-4-tert-butylcyclohexanol

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
11
Name:
HOCH2CH2OH
HOCH2CH2OH

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
12
Draw: glycerol

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
13
Name: 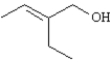


Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
14
Exhibit 17-2
Refer to the data below to answer the following question(s).
pKas of Some Phenols Y
Y
pKa
−H
9.89
m-NO2
8.28
p-NO2
7.17
m-OCH3
9.65
p-OCH3
10.21
Refer to Exhibit 17-2.How do you account for the difference in acidity between meta and para-nitrophenol?
Refer to the data below to answer the following question(s).
pKas of Some Phenols
 Y
YpKa
−H
9.89
m-NO2
8.28
p-NO2
7.17
m-OCH3
9.65
p-OCH3
10.21
Refer to Exhibit 17-2.How do you account for the difference in acidity between meta and para-nitrophenol?

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
15
Draw: 3-methyl-2-buten-1-ol

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
16
Draw: 2,4,6-trinitrophenol

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
17
Name: 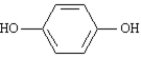


Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
18
Name: 


Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
19
Exhibit 17-4
To answer the following question(s),consider the reaction below:
Refer to Exhibit 17-4.Why is 3-methylcyclohexene the major product of this reaction instead of 1-methycyclohexene?
To answer the following question(s),consider the reaction below:

Refer to Exhibit 17-4.Why is 3-methylcyclohexene the major product of this reaction instead of 1-methycyclohexene?

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
20
Exhibit 17-2
Refer to the data below to answer the following question(s).
pKas of Some Phenols Y
Y
pKa
−H
9.89
m-NO2
8.28
p-NO2
7.17
m-OCH3
9.65
p-OCH3
10.21
Refer to Exhibit 17-2.Which of the acids in the Table has the weakest conjugate base?
Refer to the data below to answer the following question(s).
pKas of Some Phenols
 Y
YpKa
−H
9.89
m-NO2
8.28
p-NO2
7.17
m-OCH3
9.65
p-OCH3
10.21
Refer to Exhibit 17-2.Which of the acids in the Table has the weakest conjugate base?

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
21
Exhibit 17-5
To answer the following question(s),consider the reaction below:
Refer to Exhibit 17-5.Write the complete stepwise mechanism for the reaction.Show all intermediates and all electron flow with arrows.
To answer the following question(s),consider the reaction below:

Refer to Exhibit 17-5.Write the complete stepwise mechanism for the reaction.Show all intermediates and all electron flow with arrows.

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
22
Outline the synthetic steps necessary to carry out the conversion below.You may use any organic or inorganic reagents you need.Show the structures of all intermediate compounds that would probably be isolated during the course of your synthesis,and show all necessary reagents. 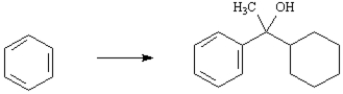


Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
23
Synthesize the following alcohol starting with cyclohexene and bromocylopentane as the only organic starting materials.Show all reagents and all intermediates in your synthesis.
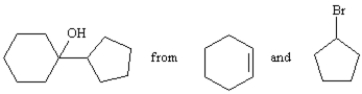


Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
24
What product is formed when the following substance is treated with CrO3 under appropriate conditions? 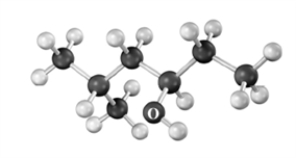
A)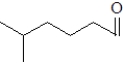
B)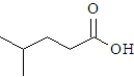
C)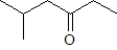
D)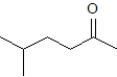
E)No reaction occurs under these conditions.

A)

B)

C)

D)

E)No reaction occurs under these conditions.

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
25
Which signal in the following proton NMR is due to an alcohol proton? 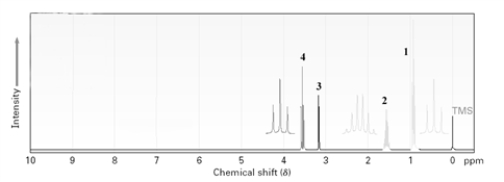
A)1
B)2
C)3
D)4
E)This cannot be determined from the data presented in the spectrum.

A)1
B)2
C)3
D)4
E)This cannot be determined from the data presented in the spectrum.

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
26
Exhibit 17-6
Consider the Grignard reaction below to answer the following question(s).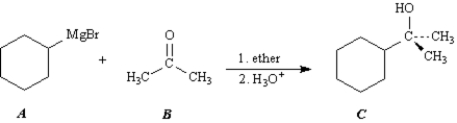
Refer to Exhibit 17-6.The nucleophile in this reaction is:
Consider the Grignard reaction below to answer the following question(s).

Refer to Exhibit 17-6.The nucleophile in this reaction is:

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
27
Exhibit 17-5
To answer the following question(s),consider the reaction below:
Refer to Exhibit 17-5.The starting material can be classified as a:
A)1° alcohol
B)2° alcohol
C)3° alcohol
D)4° alcohol
To answer the following question(s),consider the reaction below:

Refer to Exhibit 17-5.The starting material can be classified as a:
A)1° alcohol
B)2° alcohol
C)3° alcohol
D)4° alcohol

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
28
Exhibit 17-5
To answer the following question(s),consider the reaction below:
Refer to Exhibit 17-5.Provide the complete IUPAC name for the starting material in this reaction.
To answer the following question(s),consider the reaction below:

Refer to Exhibit 17-5.Provide the complete IUPAC name for the starting material in this reaction.

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
29
Give the IUPAC name for the following compound. 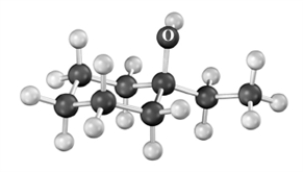


Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
30
In a proton NMR,the signal for the -OH proton
A)will generally be upfield from the corresponding alkyl signal.
B)is generally unsplit by adjacent protons.
C)is lost due to exchange with acidic impurities.
D)covers a broader chemical shift range than a phenol -OH.
A)will generally be upfield from the corresponding alkyl signal.
B)is generally unsplit by adjacent protons.
C)is lost due to exchange with acidic impurities.
D)covers a broader chemical shift range than a phenol -OH.

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
31
Exhibit 17-6
Consider the Grignard reaction below to answer the following question(s).
Refer to Exhibit 17-6.The alcohol product can be classified as a:
A)1° alcohol
B)2° alcohol
C)3° alcohol
D)4° alcohol
Consider the Grignard reaction below to answer the following question(s).

Refer to Exhibit 17-6.The alcohol product can be classified as a:
A)1° alcohol
B)2° alcohol
C)3° alcohol
D)4° alcohol

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
32
2,4,6-trinitrophenol is a stronger acid than phosphoric acid.Another name for this compound is picric acid.Explain the acidity of picric acid.

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
33
Classify the following alcohol.Atoms other than carbon and hydrogen are labeled. 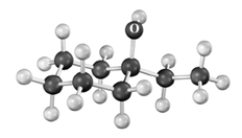
A)primary
B)secondary
C)tertiary

A)primary
B)secondary
C)tertiary

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
34
Which of the peak in the IR spectrum shown is due to the C-O stretch in an alcohol? 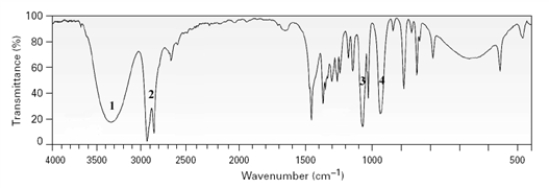
A)1
B)2
C)3
D)4

A)1
B)2
C)3
D)4

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
35
Exhibit 17-6
Consider the Grignard reaction below to answer the following question(s).
Refer to Exhibit 17-6.The electrophile in this reaction is:
Consider the Grignard reaction below to answer the following question(s).

Refer to Exhibit 17-6.The electrophile in this reaction is:

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
36
Exhibit 17-5
To answer the following question(s),consider the reaction below:
Refer to Exhibit 17-5.The conversion of an alcohol into an alkyl chloride by reaction with SOC12 is an example of:
A)an E1 process
B)an SN1 process
C)an E2 process
D)an SN2 process
To answer the following question(s),consider the reaction below:

Refer to Exhibit 17-5.The conversion of an alcohol into an alkyl chloride by reaction with SOC12 is an example of:
A)an E1 process
B)an SN1 process
C)an E2 process
D)an SN2 process

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
37
Which of the following would have the highest boiling point?
A)butane
B)1- butanol
C)chloropropane
D)pentane
A)butane
B)1- butanol
C)chloropropane
D)pentane

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
38
Which of the following would be the most acidic?
A)p-nitrophenol
B)phenol
C)p-chlorophenol
D)p-methylphenol
A)p-nitrophenol
B)phenol
C)p-chlorophenol
D)p-methylphenol

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
39
Propose a synthesis of Dimestrol starting from p-methoxypropiophenone as the only source of carbon. 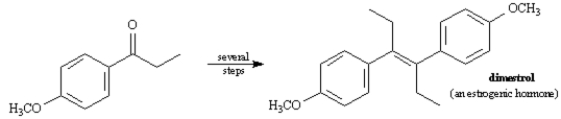


Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
40
Acid-catalyzed dehydration of 2,2-dimethylcyclohexanol yields 1,2-dimethylcyclohexene as one of the major products.Write the complete stepwise mechanism for this reaction.Show all electron flow with arrows and show all intermediate structures. 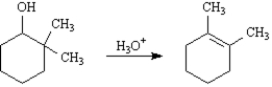


Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
41
Draw the resonance forms of 4-nitro-m-cresol.

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
42
Draw the mechanism for the acid-catalyzed dehydration of 2-methyl-2-pentanol to yield 2-methyl-1-butene.

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
43
Draw the mechanism of the E2 reaction involving the dehydration of 3-methyl-1-cyclohexanol,when reacting with POCl3 in pyridine.

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
44
The mass spectrum for the following compound would have a peak at which m/z ratio due to dehydration? Atoms other than carbon and hydrogen are labeled. 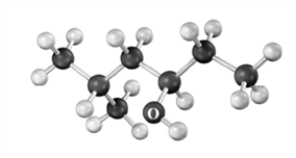
A)116
B)98
C)87
D)59

A)116
B)98
C)87
D)59

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck



