Deck 15: Benzene and Aromaticity
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/47
Play
Full screen (f)
Deck 15: Benzene and Aromaticity
1
Exhibit 15-2
Answer the following question(s) concerning sulfathiazole,below.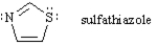
Refer to Exhibit 15-2.What reactivity do you predict for sulfathiazole?
Answer the following question(s) concerning sulfathiazole,below.
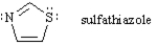
Refer to Exhibit 15-2.What reactivity do you predict for sulfathiazole?
Sulfathiazole is predicted to have aromatic reactivity since it is cyclic,planar,conjugated and has six π-electrons.
2
Name: 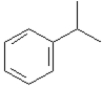

(1-methylethyl) benzene or isopropylbenzene or cumene
3
Name: 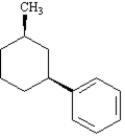

1-((1S,3R)-3-methylcyclohexyl)benzene
4
Draw: 3,5-dimethylbenzoic acid

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
5
Exhibit 15-3
Refer to the data below to answer the following question(s).
The 1H NMR spectrum of [14]annulene at −60°C shows two signals,one at 0 ppm and one at 7.6 ppm,with an area ratio of 5:2.![Exhibit 15-3 Refer to the data below to answer the following question(s). The <sup>1</sup>H NMR spectrum of [14]annulene at −60°C shows two signals,one at 0 ppm and one at 7.6 ppm,with an area ratio of 5:2. Refer to Exhibit 15-3.Is [14]annulene aromatic?](https://d2lvgg3v3hfg70.cloudfront.net/TB4944/11eab917_1508_405a_99e6_37d71c194b26_TB4944_00_TB4944_00_TB4944_00.jpg)
Refer to Exhibit 15-3.Is [14]annulene aromatic?
Refer to the data below to answer the following question(s).
The 1H NMR spectrum of [14]annulene at −60°C shows two signals,one at 0 ppm and one at 7.6 ppm,with an area ratio of 5:2.
![Exhibit 15-3 Refer to the data below to answer the following question(s). The <sup>1</sup>H NMR spectrum of [14]annulene at −60°C shows two signals,one at 0 ppm and one at 7.6 ppm,with an area ratio of 5:2. Refer to Exhibit 15-3.Is [14]annulene aromatic?](https://d2lvgg3v3hfg70.cloudfront.net/TB4944/11eab917_1508_405a_99e6_37d71c194b26_TB4944_00_TB4944_00_TB4944_00.jpg)
Refer to Exhibit 15-3.Is [14]annulene aromatic?

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
6
Name: 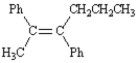


Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
7
Draw: p-chlorobenzaldehyde

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
8
Exhibit 15-2
Answer the following question(s) concerning sulfathiazole,below.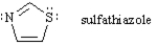
Refer to Exhibit 15-2.What is the hybridization of the nitrogen atom in sulfathiazole?
Answer the following question(s) concerning sulfathiazole,below.
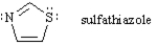
Refer to Exhibit 15-2.What is the hybridization of the nitrogen atom in sulfathiazole?

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
9
Exhibit 15-4
Consider the data below to answer the following question(s).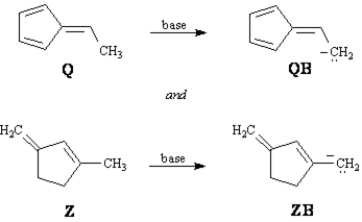
Refer to Exhibit 15-4.Conjugate bases QB and ZB are both resonance stabilized.Draw the indicated number of resonance forms for QB and ZB.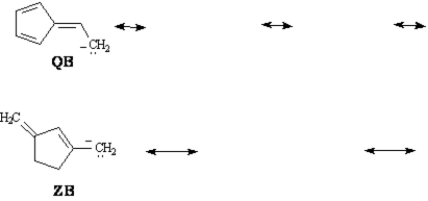
Consider the data below to answer the following question(s).

Refer to Exhibit 15-4.Conjugate bases QB and ZB are both resonance stabilized.Draw the indicated number of resonance forms for QB and ZB.


Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
10
Draw: 1-phenyl-3-methylpentane

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
11
Exhibit 15-3
Refer to the data below to answer the following question(s).
The 1H NMR spectrum of [14]annulene at −60°C shows two signals,one at 0 ppm and one at 7.6 ppm,with an area ratio of 5:2.![Exhibit 15-3 Refer to the data below to answer the following question(s). The <sup>1</sup>H NMR spectrum of [14]annulene at −60°C shows two signals,one at 0 ppm and one at 7.6 ppm,with an area ratio of 5:2. Refer to Exhibit 15-3.Draw in the protons on the [14]annulene skeleton provided below which are responsible for the <sup>1</sup>H NMR signal at 0 ppm.](https://d2lvgg3v3hfg70.cloudfront.net/TB4944/11eab917_1508_405a_99e6_37d71c194b26_TB4944_00_TB4944_00_TB4944_00.jpg)
Refer to Exhibit 15-3.Draw in the protons on the [14]annulene skeleton provided below which are responsible for the 1H NMR signal at 0 ppm.![Exhibit 15-3 Refer to the data below to answer the following question(s). The <sup>1</sup>H NMR spectrum of [14]annulene at −60°C shows two signals,one at 0 ppm and one at 7.6 ppm,with an area ratio of 5:2. Refer to Exhibit 15-3.Draw in the protons on the [14]annulene skeleton provided below which are responsible for the <sup>1</sup>H NMR signal at 0 ppm.](https://d2lvgg3v3hfg70.cloudfront.net/TB4944/11eab917_1508_405b_99e6_4f9c43851950_TB4944_00.jpg)
Refer to the data below to answer the following question(s).
The 1H NMR spectrum of [14]annulene at −60°C shows two signals,one at 0 ppm and one at 7.6 ppm,with an area ratio of 5:2.
![Exhibit 15-3 Refer to the data below to answer the following question(s). The <sup>1</sup>H NMR spectrum of [14]annulene at −60°C shows two signals,one at 0 ppm and one at 7.6 ppm,with an area ratio of 5:2. Refer to Exhibit 15-3.Draw in the protons on the [14]annulene skeleton provided below which are responsible for the <sup>1</sup>H NMR signal at 0 ppm.](https://d2lvgg3v3hfg70.cloudfront.net/TB4944/11eab917_1508_405a_99e6_37d71c194b26_TB4944_00_TB4944_00_TB4944_00.jpg)
Refer to Exhibit 15-3.Draw in the protons on the [14]annulene skeleton provided below which are responsible for the 1H NMR signal at 0 ppm.
![Exhibit 15-3 Refer to the data below to answer the following question(s). The <sup>1</sup>H NMR spectrum of [14]annulene at −60°C shows two signals,one at 0 ppm and one at 7.6 ppm,with an area ratio of 5:2. Refer to Exhibit 15-3.Draw in the protons on the [14]annulene skeleton provided below which are responsible for the <sup>1</sup>H NMR signal at 0 ppm.](https://d2lvgg3v3hfg70.cloudfront.net/TB4944/11eab917_1508_405b_99e6_4f9c43851950_TB4944_00.jpg)

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
12
Name: 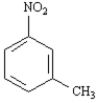


Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
13
Name: 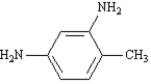


Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
14
Most alkyl halides are nonpolar covalent compounds and,therefore,are soluble in nonpolar solvents and insoluble in water.Cycloheptatrienyl bromide is an unusual alkyl halide in that it is insoluble in nonpolar solvents,but is readily soluble in water! This behavior is consistent with cycloheptatrienyl bromide being an ionic compound.Why does cycloheptatrienyl bromide exist as an ionic compound? Explain by comparing the covalent structure to the ionic structure. 


Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
15
Draw: p-bromoaniline

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
16
Exhibit 15-3
Refer to the data below to answer the following question(s).
The 1H NMR spectrum of [14]annulene at −60°C shows two signals,one at 0 ppm and one at 7.6 ppm,with an area ratio of 5:2.![Exhibit 15-3 Refer to the data below to answer the following question(s). The <sup>1</sup>H NMR spectrum of [14]annulene at −60°C shows two signals,one at 0 ppm and one at 7.6 ppm,with an area ratio of 5:2. Refer to Exhibit 15-3.Explain why these protons have absorption at such high fields.](https://d2lvgg3v3hfg70.cloudfront.net/TB4944/11eab917_1508_405a_99e6_37d71c194b26_TB4944_00_TB4944_00_TB4944_00.jpg)
Refer to Exhibit 15-3.Explain why these protons have absorption at such high fields.
Refer to the data below to answer the following question(s).
The 1H NMR spectrum of [14]annulene at −60°C shows two signals,one at 0 ppm and one at 7.6 ppm,with an area ratio of 5:2.
![Exhibit 15-3 Refer to the data below to answer the following question(s). The <sup>1</sup>H NMR spectrum of [14]annulene at −60°C shows two signals,one at 0 ppm and one at 7.6 ppm,with an area ratio of 5:2. Refer to Exhibit 15-3.Explain why these protons have absorption at such high fields.](https://d2lvgg3v3hfg70.cloudfront.net/TB4944/11eab917_1508_405a_99e6_37d71c194b26_TB4944_00_TB4944_00_TB4944_00.jpg)
Refer to Exhibit 15-3.Explain why these protons have absorption at such high fields.

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
17
Draw: o-chlorophenol

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
18
Draw: m-fluoronitrobenzene

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
19
Exhibit 15-4
Consider the data below to answer the following question(s).
Refer to Exhibit 15-4.Which of the compounds above,Q or Z,would you predict to be most acidic? Explain your answer.
Consider the data below to answer the following question(s).

Refer to Exhibit 15-4.Which of the compounds above,Q or Z,would you predict to be most acidic? Explain your answer.

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
20
Exhibit 15-2
Answer the following question(s) concerning sulfathiazole,below.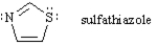
Refer to Exhibit 15-2.Assuming that the sulfur atom is sp2-hybridized,how many π-electrons are there in the sulfathiazole ring?
Answer the following question(s) concerning sulfathiazole,below.
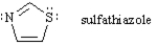
Refer to Exhibit 15-2.Assuming that the sulfur atom is sp2-hybridized,how many π-electrons are there in the sulfathiazole ring?

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
21
Draw a picture of the π orbitals in furan (shown below).Is furan aromatic? 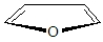


Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
22
Exhibit 15-5
Consider the data below to answer the following question(s).
C9H12;1H NMR:
7.18 δ (broad singlet,5H)
2.55 δ (triplet,2H)
1.70 δ (sextet,2H)
0.9 δ (triplet,3H)
Refer to Exhibit 15-5.Describe the signal at 7.18 δ in terms of its splitting,integration and chemical shift.
Consider the data below to answer the following question(s).
C9H12;1H NMR:
7.18 δ (broad singlet,5H)
2.55 δ (triplet,2H)
1.70 δ (sextet,2H)
0.9 δ (triplet,3H)
Refer to Exhibit 15-5.Describe the signal at 7.18 δ in terms of its splitting,integration and chemical shift.

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
23
Exhibit 15-5
Consider the data below to answer the following question(s).
C9H12;1H NMR:
7.18 δ (broad singlet,5H)
2.55 δ (triplet,2H)
1.70 δ (sextet,2H)
0.9 δ (triplet,3H)
Refer to Exhibit 15-5.Describe the signal at 2.55 δ in terms of its splitting,integration,and chemical shift.
Consider the data below to answer the following question(s).
C9H12;1H NMR:
7.18 δ (broad singlet,5H)
2.55 δ (triplet,2H)
1.70 δ (sextet,2H)
0.9 δ (triplet,3H)
Refer to Exhibit 15-5.Describe the signal at 2.55 δ in terms of its splitting,integration,and chemical shift.

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
24
Explain in terms of MO theory,why systems with 4n + 2 π electrons are aromatic and with 4n π electrons are antiaromatic.

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
25
Exhibit 15-5
Consider the data below to answer the following question(s).
C9H12;1H NMR:
7.18 δ (broad singlet,5H)
2.55 δ (triplet,2H)
1.70 δ (sextet,2H)
0.9 δ (triplet,3H)
Refer to Exhibit 15-5.Describe the signal at 0.9 δ in terms of its splitting,integration and chemical shift.
Consider the data below to answer the following question(s).
C9H12;1H NMR:
7.18 δ (broad singlet,5H)
2.55 δ (triplet,2H)
1.70 δ (sextet,2H)
0.9 δ (triplet,3H)
Refer to Exhibit 15-5.Describe the signal at 0.9 δ in terms of its splitting,integration and chemical shift.

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
26
Exhibit 15-7
Consider the data below to answer the following question(s).
C7H7ClO;IR absorption at 810 cm−1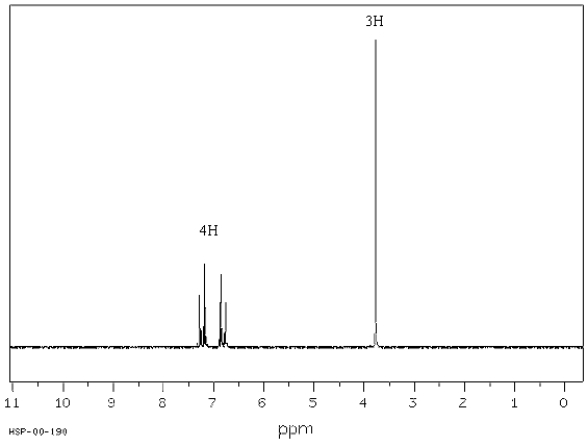 Spectrum obtained from: SDBSWeb: http://www.aist.go.jp/RIODB/SDBS/
Spectrum obtained from: SDBSWeb: http://www.aist.go.jp/RIODB/SDBS/
Refer to Exhibit 15-7.Calculate the degrees of unsaturation for this compound.
Consider the data below to answer the following question(s).
C7H7ClO;IR absorption at 810 cm−1
 Spectrum obtained from: SDBSWeb: http://www.aist.go.jp/RIODB/SDBS/
Spectrum obtained from: SDBSWeb: http://www.aist.go.jp/RIODB/SDBS/Refer to Exhibit 15-7.Calculate the degrees of unsaturation for this compound.

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
27
Consider the following compound.Atoms other than carbon and hydrogen are labeled.  Which of the following is not valid?
Which of the following is not valid?
A)naming of the parent compound,benzene.
B)1,4-disubstitution is present.
C)para prefix applicable.
D)classified as aromatic.
 Which of the following is not valid?
Which of the following is not valid?A)naming of the parent compound,benzene.
B)1,4-disubstitution is present.
C)para prefix applicable.
D)classified as aromatic.

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
28
What is the structure of a hydrocarbon that shows a molecular ion at m/z = 182 in the mass spectrum and has the following 1H NMR spectrum?
7.2 δ,singlet,5H
2.9 δ,singlet,2H
7.2 δ,singlet,5H
2.9 δ,singlet,2H

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
29
Give the IUPAC name for: 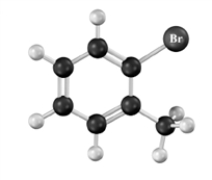


Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
30
Exhibit 15-7
Consider the data below to answer the following question(s).
C7H7ClO;IR absorption at 810 cm−1 Spectrum obtained from: SDBSWeb: http://www.aist.go.jp/RIODB/SDBS/
Spectrum obtained from: SDBSWeb: http://www.aist.go.jp/RIODB/SDBS/
Refer to Exhibit 15-7.What is the significance of an IR absorption at 810 cm−1?
Consider the data below to answer the following question(s).
C7H7ClO;IR absorption at 810 cm−1
 Spectrum obtained from: SDBSWeb: http://www.aist.go.jp/RIODB/SDBS/
Spectrum obtained from: SDBSWeb: http://www.aist.go.jp/RIODB/SDBS/Refer to Exhibit 15-7.What is the significance of an IR absorption at 810 cm−1?

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
31
How many monosubstituted products would you expect for the bromination of naphthalene?
A)2
B)4
C)6
D)8
A)2
B)4
C)6
D)8

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
32
Exhibit 15-5
Consider the data below to answer the following question(s).
C9H12;1H NMR:
7.18 δ (broad singlet,5H)
2.55 δ (triplet,2H)
1.70 δ (sextet,2H)
0.9 δ (triplet,3H)
Refer to Exhibit 15-5.Describe the signal at 1.70 δ in terms of its splitting,integration and chemical shift.
Consider the data below to answer the following question(s).
C9H12;1H NMR:
7.18 δ (broad singlet,5H)
2.55 δ (triplet,2H)
1.70 δ (sextet,2H)
0.9 δ (triplet,3H)
Refer to Exhibit 15-5.Describe the signal at 1.70 δ in terms of its splitting,integration and chemical shift.

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
33
Exhibit 15-6
Consider the data below to answer the following question(s).
C9H12;1H NMR:
6.65 δ (singlet,3H)
2.25 δ (singlet,9H)
Refer to Exhibit 15-6.Describe the signal at 6.65 δ in terms of its splitting,integration and chemical shift.
Consider the data below to answer the following question(s).
C9H12;1H NMR:
6.65 δ (singlet,3H)
2.25 δ (singlet,9H)
Refer to Exhibit 15-6.Describe the signal at 6.65 δ in terms of its splitting,integration and chemical shift.

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
34
Draw the resonance structures of cyclooctatetraene.

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
35
Draw the MO energy diagram for the cycloheptatrienyl anion and classify the anion as aromatic or antiaromatic.

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
36
Exhibit 15-7
Consider the data below to answer the following question(s).
C7H7ClO;IR absorption at 810 cm−1 Spectrum obtained from: SDBSWeb: http://www.aist.go.jp/RIODB/SDBS/
Spectrum obtained from: SDBSWeb: http://www.aist.go.jp/RIODB/SDBS/
Refer to Exhibit 15-7.Describe the signal at 3.7 δ in terms of its splitting,integration and chemical shift.
Consider the data below to answer the following question(s).
C7H7ClO;IR absorption at 810 cm−1
 Spectrum obtained from: SDBSWeb: http://www.aist.go.jp/RIODB/SDBS/
Spectrum obtained from: SDBSWeb: http://www.aist.go.jp/RIODB/SDBS/Refer to Exhibit 15-7.Describe the signal at 3.7 δ in terms of its splitting,integration and chemical shift.

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
37
Exhibit 15-6
Consider the data below to answer the following question(s).
C9H12;1H NMR:
6.65 δ (singlet,3H)
2.25 δ (singlet,9H)
Refer to Exhibit 15-6.Describe the signal at 2.25 δ in terms of its splitting,integration and chemical shift.
Consider the data below to answer the following question(s).
C9H12;1H NMR:
6.65 δ (singlet,3H)
2.25 δ (singlet,9H)
Refer to Exhibit 15-6.Describe the signal at 2.25 δ in terms of its splitting,integration and chemical shift.

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
38
Exhibit 15-6
Consider the data below to answer the following question(s).
C9H12;1H NMR:
6.65 δ (singlet,3H)
2.25 δ (singlet,9H)
Refer to Exhibit 15-6.Propose a structure for this compound.
Consider the data below to answer the following question(s).
C9H12;1H NMR:
6.65 δ (singlet,3H)
2.25 δ (singlet,9H)
Refer to Exhibit 15-6.Propose a structure for this compound.

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
39
Exhibit 15-7
Consider the data below to answer the following question(s).
C7H7ClO;IR absorption at 810 cm−1 Spectrum obtained from: SDBSWeb: http://www.aist.go.jp/RIODB/SDBS/
Spectrum obtained from: SDBSWeb: http://www.aist.go.jp/RIODB/SDBS/
Refer to Exhibit 15-7.Propose a structure for this compound.
Consider the data below to answer the following question(s).
C7H7ClO;IR absorption at 810 cm−1
 Spectrum obtained from: SDBSWeb: http://www.aist.go.jp/RIODB/SDBS/
Spectrum obtained from: SDBSWeb: http://www.aist.go.jp/RIODB/SDBS/Refer to Exhibit 15-7.Propose a structure for this compound.

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
40
Exhibit 15-5
Consider the data below to answer the following question(s).
C9H12;1H NMR:
7.18 δ (broad singlet,5H)
2.55 δ (triplet,2H)
1.70 δ (sextet,2H)
0.9 δ (triplet,3H)
Refer to Exhibit 15-5.Propose a structure for this compound.
Consider the data below to answer the following question(s).
C9H12;1H NMR:
7.18 δ (broad singlet,5H)
2.55 δ (triplet,2H)
1.70 δ (sextet,2H)
0.9 δ (triplet,3H)
Refer to Exhibit 15-5.Propose a structure for this compound.

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
41
Draw the different resonance forms of phenanthrene.

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
42
What m/z ratio range might be common to many substituted benzene compounds?
A)72 - 77 ppm
B)26 - 38 ppm
C)81 - 88 ppm
D)less than 59 ppm
A)72 - 77 ppm
B)26 - 38 ppm
C)81 - 88 ppm
D)less than 59 ppm

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
43
Draw the resonance forms of indene.

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
44
Which of the following does not correctly characterize this compound represented by the molecular model shown below? Atoms other than carbon and hydrogen are labeled. 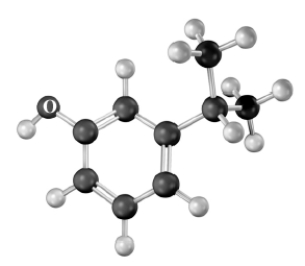
A)slightly acidic
B)named as isopropyl o-cresol
C)contains aryl,alkyl and benzylic proton
D)would produce a signal due to dehydration in a mass spectrum
E)All of the above characterize this compound.

A)slightly acidic
B)named as isopropyl o-cresol
C)contains aryl,alkyl and benzylic proton
D)would produce a signal due to dehydration in a mass spectrum
E)All of the above characterize this compound.

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
45
Which of the following aromatic compounds will have largest number of signals in a 13CNMR?
A)1,4-dimethylbenzene (p-methyl toluene)
B)1,3,5-trimethylbenzene (mesitylene)
C)1,2,4,5-tetramethylbenzene
D)All produce the same number of signals.
A)1,4-dimethylbenzene (p-methyl toluene)
B)1,3,5-trimethylbenzene (mesitylene)
C)1,2,4,5-tetramethylbenzene
D)All produce the same number of signals.

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
46
Which of the choices arranges numbered protons in the following substance in order of increasing downfield chemical shift in a proton NMR? Atoms other than carbon and hydrogen are labeled. 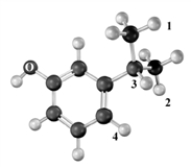
A)1 = 2 < 3 < 4
B)4 < 3 < 2 = 1
C)4 = 3 < 2 =1
D)1 = 2 < 4 < 3

A)1 = 2 < 3 < 4
B)4 < 3 < 2 = 1
C)4 = 3 < 2 =1
D)1 = 2 < 4 < 3

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
47
Draw the different resonance forms of anthracene.

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck



