Deck 11: Reactions of Alkyl Halides: Nucleophilic Substitutions and Eliminations
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/34
Play
Full screen (f)
Deck 11: Reactions of Alkyl Halides: Nucleophilic Substitutions and Eliminations
1
Exhibit 11-7
To answer the following question(s) consider the data below:
Reaction of bromomethane with sodium hydroxide in water forms methanol.If sodium iodide is added to the reaction mixture,the rate of methanol formation is dramatically increased (i.e.sodium iodide is a catalyst).
Refer to Exhibit 11-7.Write a reaction pathway that accounts for the effect of added NaI.
To answer the following question(s) consider the data below:
Reaction of bromomethane with sodium hydroxide in water forms methanol.If sodium iodide is added to the reaction mixture,the rate of methanol formation is dramatically increased (i.e.sodium iodide is a catalyst).
Refer to Exhibit 11-7.Write a reaction pathway that accounts for the effect of added NaI.
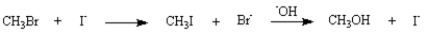
2
Exhibit 11-6
Consider the reaction below to answer the following question(s).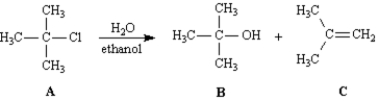
Refer to Exhibit 11-6.Compound C is the:
A)SN2 product
B)SN1 product
C)E2 product
D)E1 product
Consider the reaction below to answer the following question(s).

Refer to Exhibit 11-6.Compound C is the:
A)SN2 product
B)SN1 product
C)E2 product
D)E1 product
E1 product
3
Exhibit 11-4
Consider the pair of reactions below to answer the following question(s).
Refer to Exhibit 11-4.The alkyl bromide starting materials in these reactions are classified as:
A)3°
B)2°
C)1°
D)4°
Consider the pair of reactions below to answer the following question(s).
Refer to Exhibit 11-4.The alkyl bromide starting materials in these reactions are classified as:
A)3°
B)2°
C)1°
D)4°
1°
4
Exhibit 11-7
To answer the following question(s) consider the data below:
Reaction of bromomethane with sodium hydroxide in water forms methanol.If sodium iodide is added to the reaction mixture,the rate of methanol formation is dramatically increased (i.e.sodium iodide is a catalyst).
Refer to Exhibit 11-7.The mechanism involved in the reaction of CH3Br and NaOH is:
A)SN1
B)SN2
C)E1
D)E2
To answer the following question(s) consider the data below:
Reaction of bromomethane with sodium hydroxide in water forms methanol.If sodium iodide is added to the reaction mixture,the rate of methanol formation is dramatically increased (i.e.sodium iodide is a catalyst).
Refer to Exhibit 11-7.The mechanism involved in the reaction of CH3Br and NaOH is:
A)SN1
B)SN2
C)E1
D)E2

Unlock Deck
Unlock for access to all 34 flashcards in this deck.
Unlock Deck
k this deck
5
Exhibit 11-9
Consider the reaction below to answer the following question(s).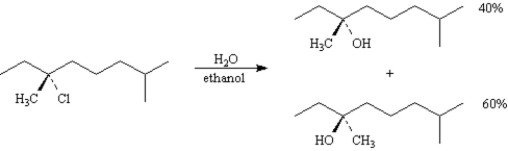
Refer to Exhibit 11-9.Write the complete stepwise mechanism for this reaction.Clearly show the formation of both products.Show all electron flow with arrows and draw all intermediate structures.
Consider the reaction below to answer the following question(s).

Refer to Exhibit 11-9.Write the complete stepwise mechanism for this reaction.Clearly show the formation of both products.Show all electron flow with arrows and draw all intermediate structures.

Unlock Deck
Unlock for access to all 34 flashcards in this deck.
Unlock Deck
k this deck
6
Exhibit 11-7
To answer the following question(s) consider the data below:
Reaction of bromomethane with sodium hydroxide in water forms methanol.If sodium iodide is added to the reaction mixture,the rate of methanol formation is dramatically increased (i.e.sodium iodide is a catalyst).
Would you expect the same catalytic effect on this reaction if you added NaCl instead? Explain your answer.
To answer the following question(s) consider the data below:
Reaction of bromomethane with sodium hydroxide in water forms methanol.If sodium iodide is added to the reaction mixture,the rate of methanol formation is dramatically increased (i.e.sodium iodide is a catalyst).
Would you expect the same catalytic effect on this reaction if you added NaCl instead? Explain your answer.

Unlock Deck
Unlock for access to all 34 flashcards in this deck.
Unlock Deck
k this deck
7
Exhibit 11-11
Consider the reaction below to answer the following question(s).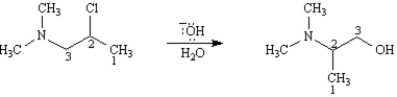
Refer to Exhibit 11-11.List all chirality centers in the starting material by number.
Consider the reaction below to answer the following question(s).

Refer to Exhibit 11-11.List all chirality centers in the starting material by number.

Unlock Deck
Unlock for access to all 34 flashcards in this deck.
Unlock Deck
k this deck
8
Exhibit 11-6
Consider the reaction below to answer the following question(s).
Refer to Exhibit 11-6.Compound B is the:
A)SN2 product
B)SN1 product
C)E2 product
D)E1 product
Consider the reaction below to answer the following question(s).

Refer to Exhibit 11-6.Compound B is the:
A)SN2 product
B)SN1 product
C)E2 product
D)E1 product

Unlock Deck
Unlock for access to all 34 flashcards in this deck.
Unlock Deck
k this deck
9
Exhibit 11-4
Consider the pair of reactions below to answer the following question(s).
Refer to Exhibit 11-4.Which reaction is faster?
A)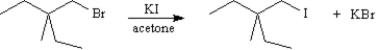 or
or
B)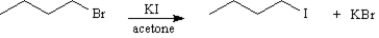
Consider the pair of reactions below to answer the following question(s).
Refer to Exhibit 11-4.Which reaction is faster?
A)
 or
orB)
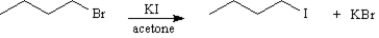

Unlock Deck
Unlock for access to all 34 flashcards in this deck.
Unlock Deck
k this deck
10
Exhibit 11-7
To answer the following question(s) consider the data below:
Reaction of bromomethane with sodium hydroxide in water forms methanol.If sodium iodide is added to the reaction mixture,the rate of methanol formation is dramatically increased (i.e.sodium iodide is a catalyst).
Refer to Exhibit 11-7.Draw a reaction energy diagram showing the two different reaction pathways (i.e.catalyzed and uncatalyzed).Indicate structures for all energy minima in the diagram.
To answer the following question(s) consider the data below:
Reaction of bromomethane with sodium hydroxide in water forms methanol.If sodium iodide is added to the reaction mixture,the rate of methanol formation is dramatically increased (i.e.sodium iodide is a catalyst).
Refer to Exhibit 11-7.Draw a reaction energy diagram showing the two different reaction pathways (i.e.catalyzed and uncatalyzed).Indicate structures for all energy minima in the diagram.

Unlock Deck
Unlock for access to all 34 flashcards in this deck.
Unlock Deck
k this deck
11
Exhibit 11-4
Consider the pair of reactions below to answer the following question(s).
Refer to Exhibit 11-4.The solvent in these reactions is:
A)nonpolar aprotic
B)polar aprotic
C)polar protic
D)nonpolar protic
Consider the pair of reactions below to answer the following question(s).
Refer to Exhibit 11-4.The solvent in these reactions is:
A)nonpolar aprotic
B)polar aprotic
C)polar protic
D)nonpolar protic

Unlock Deck
Unlock for access to all 34 flashcards in this deck.
Unlock Deck
k this deck
12
Exhibit 11-9
Consider the reaction below to answer the following question(s).![<strong>Exhibit 11-9 Consider the reaction below to answer the following question(s). Refer to Exhibit 11-9.This reaction obeys a rate law of the form:</strong> A)rate = k[RCl][CH<sub>3</sub>CH<sub>2</sub>OH] B)rate = k[RCl][H<sub>2</sub>O][CH<sub>3</sub>CH<sub>2</sub>OH] C)rate = k[RCl] D)rate = k[RCl][H<sub>2</sub>O]](https://d2lvgg3v3hfg70.cloudfront.net/TB4944/11eab917_1512_2b01_99e6_c30aa3b5d73f_TB4944_00_TB4944_00.jpg)
Refer to Exhibit 11-9.This reaction obeys a rate law of the form:
A)rate = k[RCl][CH3CH2OH]
B)rate = k[RCl][H2O][CH3CH2OH]
C)rate = k[RCl]
D)rate = k[RCl][H2O]
Consider the reaction below to answer the following question(s).
![<strong>Exhibit 11-9 Consider the reaction below to answer the following question(s). Refer to Exhibit 11-9.This reaction obeys a rate law of the form:</strong> A)rate = k[RCl][CH<sub>3</sub>CH<sub>2</sub>OH] B)rate = k[RCl][H<sub>2</sub>O][CH<sub>3</sub>CH<sub>2</sub>OH] C)rate = k[RCl] D)rate = k[RCl][H<sub>2</sub>O]](https://d2lvgg3v3hfg70.cloudfront.net/TB4944/11eab917_1512_2b01_99e6_c30aa3b5d73f_TB4944_00_TB4944_00.jpg)
Refer to Exhibit 11-9.This reaction obeys a rate law of the form:
A)rate = k[RCl][CH3CH2OH]
B)rate = k[RCl][H2O][CH3CH2OH]
C)rate = k[RCl]
D)rate = k[RCl][H2O]

Unlock Deck
Unlock for access to all 34 flashcards in this deck.
Unlock Deck
k this deck
13
Exhibit 11-6
Consider the reaction below to answer the following question(s).
Refer to Exhibit 11-6.Write the complete reaction mechanism for the formation of Compound C in this reaction.
Consider the reaction below to answer the following question(s).

Refer to Exhibit 11-6.Write the complete reaction mechanism for the formation of Compound C in this reaction.

Unlock Deck
Unlock for access to all 34 flashcards in this deck.
Unlock Deck
k this deck
14
Exhibit 11-6
Consider the reaction below to answer the following question(s).
Refer to Exhibit 11-6.The substrate in the reaction is:
Consider the reaction below to answer the following question(s).

Refer to Exhibit 11-6.The substrate in the reaction is:

Unlock Deck
Unlock for access to all 34 flashcards in this deck.
Unlock Deck
k this deck
15
Exhibit 11-4
Consider the pair of reactions below to answer the following question(s).
Refer to Exhibit 11-4.The nucleophile in these reactions is:
A)K+
B)alkyl group
C)Br−
D)I−
Consider the pair of reactions below to answer the following question(s).
Refer to Exhibit 11-4.The nucleophile in these reactions is:
A)K+
B)alkyl group
C)Br−
D)I−

Unlock Deck
Unlock for access to all 34 flashcards in this deck.
Unlock Deck
k this deck
16
Exhibit 11-7
To answer the following question(s) consider the data below:
Reaction of bromomethane with sodium hydroxide in water forms methanol.If sodium iodide is added to the reaction mixture,the rate of methanol formation is dramatically increased (i.e.sodium iodide is a catalyst).
Refer to Exhibit 11-7.Explain why adding NaI increases the reaction rate.
To answer the following question(s) consider the data below:
Reaction of bromomethane with sodium hydroxide in water forms methanol.If sodium iodide is added to the reaction mixture,the rate of methanol formation is dramatically increased (i.e.sodium iodide is a catalyst).
Refer to Exhibit 11-7.Explain why adding NaI increases the reaction rate.

Unlock Deck
Unlock for access to all 34 flashcards in this deck.
Unlock Deck
k this deck
17
Exhibit 11-8
Consider the reaction below to answer the following question(s):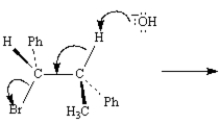
Refer to Exhibit 11-8.Draw a Newman projection of the reactive conformation of the starting material.
Consider the reaction below to answer the following question(s):

Refer to Exhibit 11-8.Draw a Newman projection of the reactive conformation of the starting material.

Unlock Deck
Unlock for access to all 34 flashcards in this deck.
Unlock Deck
k this deck
18
Exhibit 11-8
Consider the reaction below to answer the following question(s):
Refer to Exhibit 11-8.Write the product that results from the electron flow in the reaction,clearly indicating any stereochemistry.
Consider the reaction below to answer the following question(s):

Refer to Exhibit 11-8.Write the product that results from the electron flow in the reaction,clearly indicating any stereochemistry.

Unlock Deck
Unlock for access to all 34 flashcards in this deck.
Unlock Deck
k this deck
19
Exhibit 11-4
Consider the pair of reactions below to answer the following question(s).
Refer to Exhibit 11-4.The mechanism for these reactions is:
A)SN2
B)E2
C)SN1
D)E1
Consider the pair of reactions below to answer the following question(s).
Refer to Exhibit 11-4.The mechanism for these reactions is:
A)SN2
B)E2
C)SN1
D)E1

Unlock Deck
Unlock for access to all 34 flashcards in this deck.
Unlock Deck
k this deck
20
Exhibit 11-8
Consider the reaction below to answer the following question(s):
Refer to Exhibit 11-8.The mechanism of this reaction is:
A)SN1
B)SN2
C)E1
D)E2
Consider the reaction below to answer the following question(s):

Refer to Exhibit 11-8.The mechanism of this reaction is:
A)SN1
B)SN2
C)E1
D)E2

Unlock Deck
Unlock for access to all 34 flashcards in this deck.
Unlock Deck
k this deck
21
For the elimination reaction: 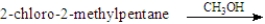 Draw the structure present at the point indicated by the asterisk (*) on the following energy diagram
Draw the structure present at the point indicated by the asterisk (*) on the following energy diagram 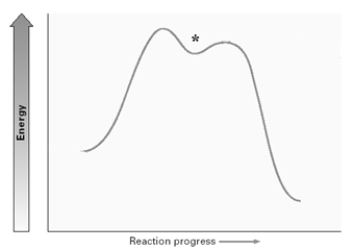
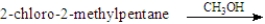 Draw the structure present at the point indicated by the asterisk (*) on the following energy diagram
Draw the structure present at the point indicated by the asterisk (*) on the following energy diagram 

Unlock Deck
Unlock for access to all 34 flashcards in this deck.
Unlock Deck
k this deck
22
Draw the E2 mechanism of the reaction involving the reduction of 2-chloro-butane to 2-butene,in the presence of KOH in ethanol.

Unlock Deck
Unlock for access to all 34 flashcards in this deck.
Unlock Deck
k this deck
23
For the elimination reaction: 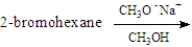 Draw the structure present at the point indicated by the asterisk (*) on the following energy diagram.
Draw the structure present at the point indicated by the asterisk (*) on the following energy diagram. 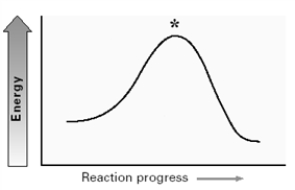
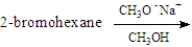 Draw the structure present at the point indicated by the asterisk (*) on the following energy diagram.
Draw the structure present at the point indicated by the asterisk (*) on the following energy diagram. 

Unlock Deck
Unlock for access to all 34 flashcards in this deck.
Unlock Deck
k this deck
24
Consider the two lines shown on the energy diagram below. 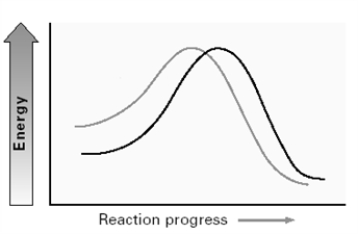 In an SN2 reaction,
In an SN2 reaction,
A)the upper left line could represent Cl- and the lower left line I-.
B)the upper left line could represent OH- and the lower left line CH3COO- .
C)the upper left line could represent H2O and the lower left line H2S.
D)the upper left line could represent (CH3)2NH and the lower left line (CH3)2N-.
 In an SN2 reaction,
In an SN2 reaction,A)the upper left line could represent Cl- and the lower left line I-.
B)the upper left line could represent OH- and the lower left line CH3COO- .
C)the upper left line could represent H2O and the lower left line H2S.
D)the upper left line could represent (CH3)2NH and the lower left line (CH3)2N-.

Unlock Deck
Unlock for access to all 34 flashcards in this deck.
Unlock Deck
k this deck
25
Draw the mechanism of the SN1 reaction of 2-chloro-2-methyl-butane with H2O.

Unlock Deck
Unlock for access to all 34 flashcards in this deck.
Unlock Deck
k this deck
26
Nucleophilic substitution and elimination reactions often compete with one another.In general,which of the following would enhance the rate of the E2 reaction over the SN2 reaction?
A)use of a weak nucleophile
B)branching at the α or β carbon
C)use of a weak base
D)All of the above would enhance the E2 rate.
A)use of a weak nucleophile
B)branching at the α or β carbon
C)use of a weak base
D)All of the above would enhance the E2 rate.

Unlock Deck
Unlock for access to all 34 flashcards in this deck.
Unlock Deck
k this deck
27
Exhibit 11-11
Consider the reaction below to answer the following question(s).
Refer to Exhibit 11-11.As indicated by the carbon numbers,during the course of this reaction the bond between the nitrogen atom and carbon 3 is broken and a bond between the nitrogen atom and carbon 2 is formed.On the structures provided below,draw arrows showing electron flow for the mechanism that accounts for these bonding changes.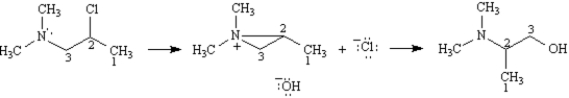
Consider the reaction below to answer the following question(s).

Refer to Exhibit 11-11.As indicated by the carbon numbers,during the course of this reaction the bond between the nitrogen atom and carbon 3 is broken and a bond between the nitrogen atom and carbon 2 is formed.On the structures provided below,draw arrows showing electron flow for the mechanism that accounts for these bonding changes.


Unlock Deck
Unlock for access to all 34 flashcards in this deck.
Unlock Deck
k this deck
28
Consider the two energy diagrams given below. A B 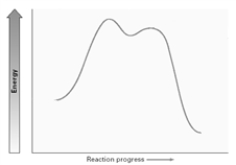
 Which of the following is correct with respect to these diagrams?
Which of the following is correct with respect to these diagrams?
A)A could represent an E2 reaction.
B)B could represent an E1 reaction.
C)A could represent an E1cb reaction.

 Which of the following is correct with respect to these diagrams?
Which of the following is correct with respect to these diagrams?A)A could represent an E2 reaction.
B)B could represent an E1 reaction.
C)A could represent an E1cb reaction.

Unlock Deck
Unlock for access to all 34 flashcards in this deck.
Unlock Deck
k this deck
29
Draw the mechanism of the SN2 reaction involving (R)-2-chloro-butane,to produce (S)-2-butanol.

Unlock Deck
Unlock for access to all 34 flashcards in this deck.
Unlock Deck
k this deck
30
Consider the two energy diagrams below for an SN2 reaction. A B 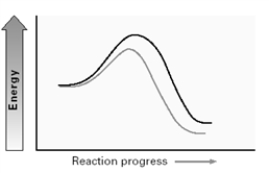
 Which of the following is correct?:
Which of the following is correct?:
A)The change in the variable shown in A affects the transition state while that of B affects the reactant.
B)The change in the variable shown in A affects the reactant while that of B affects the transition state.
C)The changes in the variables shown in both A and B affect the transition state.
D)The changes in the variables shown in both A and B affect the reactants.

 Which of the following is correct?:
Which of the following is correct?:A)The change in the variable shown in A affects the transition state while that of B affects the reactant.
B)The change in the variable shown in A affects the reactant while that of B affects the transition state.
C)The changes in the variables shown in both A and B affect the transition state.
D)The changes in the variables shown in both A and B affect the reactants.

Unlock Deck
Unlock for access to all 34 flashcards in this deck.
Unlock Deck
k this deck
31
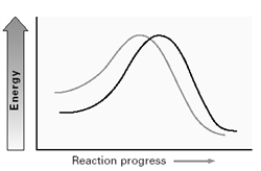
Consider the two lines shown on the energy diagram below. 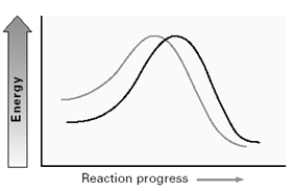 In an SN2 reaction,these lines could compare the effect of which of the following solvents?
In an SN2 reaction,these lines could compare the effect of which of the following solvents?
A)CH3OH and CH3CH2OH
B)CH3OH and H2O
C)CH3OH and CH3CN
D)DMSO and CH3CN
 In an SN2 reaction,these lines could compare the effect of which of the following solvents?
In an SN2 reaction,these lines could compare the effect of which of the following solvents?A)CH3OH and CH3CH2OH
B)CH3OH and H2O
C)CH3OH and CH3CN
D)DMSO and CH3CN

Unlock Deck
Unlock for access to all 34 flashcards in this deck.
Unlock Deck
k this deck
32
For the substitution reaction: 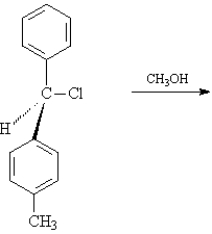 Draw the structure present at the point indicated by the asterisk (*) on the following energy diagram.Identify the reactant as R or S or neither.
Draw the structure present at the point indicated by the asterisk (*) on the following energy diagram.Identify the reactant as R or S or neither. 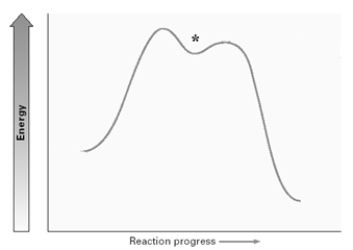
 Draw the structure present at the point indicated by the asterisk (*) on the following energy diagram.Identify the reactant as R or S or neither.
Draw the structure present at the point indicated by the asterisk (*) on the following energy diagram.Identify the reactant as R or S or neither. 

Unlock Deck
Unlock for access to all 34 flashcards in this deck.
Unlock Deck
k this deck
33
Exhibit 11-11
Consider the reaction below to answer the following question(s).
Refer to Exhibit 11-11.If the absolute configuration at carbon 2 in the starting material is R,what is the absolute configuration at carbon 2 in the product?
Consider the reaction below to answer the following question(s).

Refer to Exhibit 11-11.If the absolute configuration at carbon 2 in the starting material is R,what is the absolute configuration at carbon 2 in the product?

Unlock Deck
Unlock for access to all 34 flashcards in this deck.
Unlock Deck
k this deck
34
For the substitution reaction: 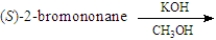 Draw the structure present at the point indicated by the asterisk (*) on the following energy diagram.
Draw the structure present at the point indicated by the asterisk (*) on the following energy diagram. 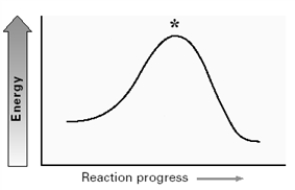
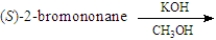 Draw the structure present at the point indicated by the asterisk (*) on the following energy diagram.
Draw the structure present at the point indicated by the asterisk (*) on the following energy diagram. 

Unlock Deck
Unlock for access to all 34 flashcards in this deck.
Unlock Deck
k this deck



