Deck 10: Organohalides
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/31
Play
Full screen (f)
Deck 10: Organohalides
1
Draw: trans-1-chloro-3-sec-butylcyclohexane
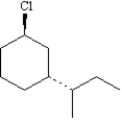
2
Exhibit 10-3
Consider the reaction below to answer the following question(s).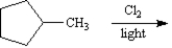
Refer to Exhibit 10-3.Label the chirality centers in the monochlorination products of methylcyclopentane with an asterisk.
Consider the reaction below to answer the following question(s).
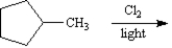
Refer to Exhibit 10-3.Label the chirality centers in the monochlorination products of methylcyclopentane with an asterisk.
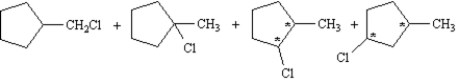
3
Exhibit 10-2
To answer the following question(s) consider the reaction below:
-The original question has been combined with question #9 as part
c.This placeholder question is here to maintain the integrity of the numbering system between the printed copy and ExamView.Therefore,it has been marked "do not use on test" in ExamView's question information dialog.As a result,this placeholder question is automatically prevented from being chosen as a test question.
To answer the following question(s) consider the reaction below:

-The original question has been combined with question #9 as part
c.This placeholder question is here to maintain the integrity of the numbering system between the printed copy and ExamView.Therefore,it has been marked "do not use on test" in ExamView's question information dialog.As a result,this placeholder question is automatically prevented from being chosen as a test question.
It appears that you are referring to a specific question from a test or textbook related to a chemical reaction, and you've provided a reference code
( ).
).
However, without the actual content of the question or the details of the reaction, it is not possible to provide an answer.
The text you've provided also indicates that the question you're referring to is a placeholder and is not meant to be used on a test. It seems to be there to maintain the numbering system between different versions of the material, such as a printed copy and an electronic copy in ExamView.
If you have the actual content of the question or details about the chemical reaction, please provide them, and I would be happy to help with an answer. Otherwise, it is not possible to answer a question without knowing what the question is.
(
 ).
).However, without the actual content of the question or the details of the reaction, it is not possible to provide an answer.
The text you've provided also indicates that the question you're referring to is a placeholder and is not meant to be used on a test. It seems to be there to maintain the numbering system between different versions of the material, such as a printed copy and an electronic copy in ExamView.
If you have the actual content of the question or details about the chemical reaction, please provide them, and I would be happy to help with an answer. Otherwise, it is not possible to answer a question without knowing what the question is.
4
The following compound has been used as a less-ozone-depleting replacement for Freons.Name this compound.
CHF2CF3
CHF2CF3

Unlock Deck
Unlock for access to all 31 flashcards in this deck.
Unlock Deck
k this deck
5
Exhibit 10-3
Consider the reaction below to answer the following question(s).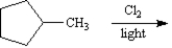
Refer to Exhibit 10-3.Draw all the monochlorination products of methylcyclopentane (ignore stereoisomers).
Consider the reaction below to answer the following question(s).
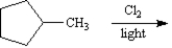
Refer to Exhibit 10-3.Draw all the monochlorination products of methylcyclopentane (ignore stereoisomers).

Unlock Deck
Unlock for access to all 31 flashcards in this deck.
Unlock Deck
k this deck
6
Exhibit 10-3
Consider the reaction below to answer the following question(s).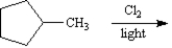
Refer to Exhibit 10-3.Tell whether radical chlorination of methylcyclopentane is an oxidation or a reduction process.
Consider the reaction below to answer the following question(s).
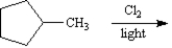
Refer to Exhibit 10-3.Tell whether radical chlorination of methylcyclopentane is an oxidation or a reduction process.

Unlock Deck
Unlock for access to all 31 flashcards in this deck.
Unlock Deck
k this deck
7
Exhibit 10-2
To answer the following question(s) consider the reaction below:

-The original question has been combined with question #9 as part
b.This placeholder question is here to maintain the integrity of the numbering system between the printed copy and ExamView.Therefore,it has been marked "do not use on test" in ExamView's question information dialog.As a result,this placeholder question is automatically prevented from being chosen as a test question.
To answer the following question(s) consider the reaction below:

-The original question has been combined with question #9 as part
b.This placeholder question is here to maintain the integrity of the numbering system between the printed copy and ExamView.Therefore,it has been marked "do not use on test" in ExamView's question information dialog.As a result,this placeholder question is automatically prevented from being chosen as a test question.

Unlock Deck
Unlock for access to all 31 flashcards in this deck.
Unlock Deck
k this deck
8
Draw the skeletal structure of the product if the following alcohol were treated with HF in pyridine.Atoms other than carbon and hydrogen are labeled. 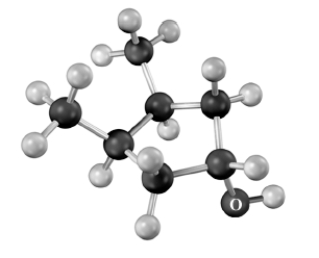


Unlock Deck
Unlock for access to all 31 flashcards in this deck.
Unlock Deck
k this deck
9
Rank the following compounds in order of increasing oxidation level.Place the number rank (1 = lowest;4 = highest) in the blank below the structure. 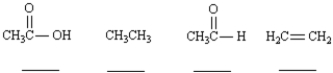


Unlock Deck
Unlock for access to all 31 flashcards in this deck.
Unlock Deck
k this deck
10
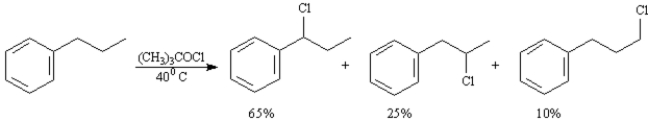
Exhibit 10-2
To answer the following question(s) consider the reaction below:
A)When propylbenzene reacts with tert-butylhypochlorite three monochlorinated products are formed in the ratios indicated.Calculate a reactivity order for each type of hydrogen atom in propylbenzene.
B)The reaction of propylbenzene with tert-butylhypochlorite proceeds by a radical substitution pathway.Draw the structure of the radical intermediate leading to each product.
C)Based on your answers to the two questions above explain why (1-chloropropyl)benzene is the major product of this reaction.
To answer the following question(s) consider the reaction below:

A)When propylbenzene reacts with tert-butylhypochlorite three monochlorinated products are formed in the ratios indicated.Calculate a reactivity order for each type of hydrogen atom in propylbenzene.
B)The reaction of propylbenzene with tert-butylhypochlorite proceeds by a radical substitution pathway.Draw the structure of the radical intermediate leading to each product.
C)Based on your answers to the two questions above explain why (1-chloropropyl)benzene is the major product of this reaction.

Unlock Deck
Unlock for access to all 31 flashcards in this deck.
Unlock Deck
k this deck
11
In the discussion on relative reactivity of alkane hydrogens towards radical chlorination,we showed that the relative rate of 2° to 1° hydrogen atom abstraction is 3.5 : 1 for butane.Calculate the relative amounts of 1-chloropropane and 2-chloropropane obtained by the radical chlorination of propane,using this relative rate of reactivity. 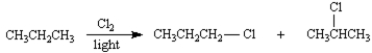
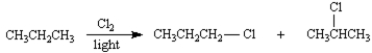

Unlock Deck
Unlock for access to all 31 flashcards in this deck.
Unlock Deck
k this deck
12
Predict the product of the following reaction. 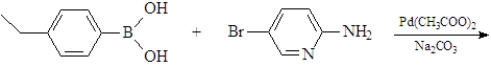


Unlock Deck
Unlock for access to all 31 flashcards in this deck.
Unlock Deck
k this deck
13
Draw: (S)-2-bromobutane

Unlock Deck
Unlock for access to all 31 flashcards in this deck.
Unlock Deck
k this deck
14
Draw: 1,2-dichloro-1,1,2,2-tetrafluoroethane (Cryofluorane)

Unlock Deck
Unlock for access to all 31 flashcards in this deck.
Unlock Deck
k this deck
15
Draw: 3-iodopropene

Unlock Deck
Unlock for access to all 31 flashcards in this deck.
Unlock Deck
k this deck
16
Exhibit 10-4
Consider the reaction below to answer the following question(s).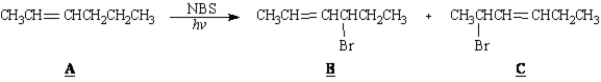
Refer to Exhibit 10-4.Draw the resonance forms of the allylic radical intermediate that accounts for the formation of B and C.
Consider the reaction below to answer the following question(s).
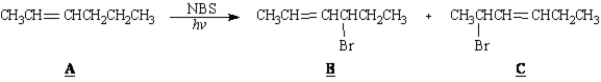
Refer to Exhibit 10-4.Draw the resonance forms of the allylic radical intermediate that accounts for the formation of B and C.

Unlock Deck
Unlock for access to all 31 flashcards in this deck.
Unlock Deck
k this deck
17
Exhibit 10-4
Consider the reaction below to answer the following question(s).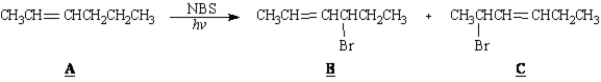
Refer to Exhibit 10-4.Place asterisks(*) at all allylic positions in compound A.
Consider the reaction below to answer the following question(s).
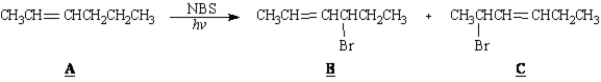
Refer to Exhibit 10-4.Place asterisks(*) at all allylic positions in compound A.

Unlock Deck
Unlock for access to all 31 flashcards in this deck.
Unlock Deck
k this deck
18
Exhibit 10-2
To answer the following question(s) consider the reaction below:
Refer to Exhibit 10-2.Are any of the products of this reaction chiral? If so draw them and label the chirality center with an asterisk.
To answer the following question(s) consider the reaction below:

Refer to Exhibit 10-2.Are any of the products of this reaction chiral? If so draw them and label the chirality center with an asterisk.

Unlock Deck
Unlock for access to all 31 flashcards in this deck.
Unlock Deck
k this deck
19
Exhibit 10-4
Consider the reaction below to answer the following question(s).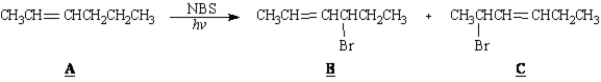
Refer to Exhibit 10-4.D and E,below,are minor products in this reaction.Explain why.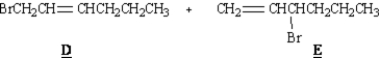
Consider the reaction below to answer the following question(s).
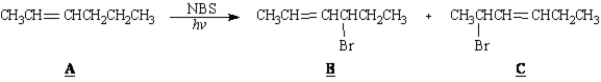
Refer to Exhibit 10-4.D and E,below,are minor products in this reaction.Explain why.
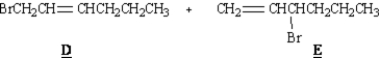

Unlock Deck
Unlock for access to all 31 flashcards in this deck.
Unlock Deck
k this deck
20
Exhibit 10-2
To answer the following question(s) consider the reaction below:
Refer to Exhibit 10-2.Will the product mixture of this reaction display optical activity?
To answer the following question(s) consider the reaction below:

Refer to Exhibit 10-2.Will the product mixture of this reaction display optical activity?

Unlock Deck
Unlock for access to all 31 flashcards in this deck.
Unlock Deck
k this deck
21
Consider the following reaction. 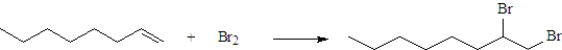 This reaction is classified as:
This reaction is classified as:
A)an oxidation.
B)a reduction.
C)neither an oxidation nor a reduction.
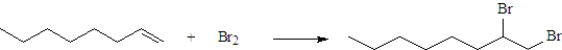 This reaction is classified as:
This reaction is classified as:A)an oxidation.
B)a reduction.
C)neither an oxidation nor a reduction.

Unlock Deck
Unlock for access to all 31 flashcards in this deck.
Unlock Deck
k this deck
22
Draw the mechanism of the allylic bromination of 1-cyclopentene.

Unlock Deck
Unlock for access to all 31 flashcards in this deck.
Unlock Deck
k this deck
23
Which of the following correctly orders oxidation levels?
A)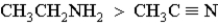
B)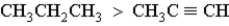
C)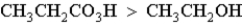
D)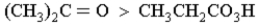
A)
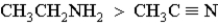
B)
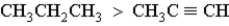
C)
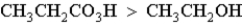
D)
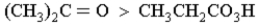

Unlock Deck
Unlock for access to all 31 flashcards in this deck.
Unlock Deck
k this deck
24
Draw the mechanism of the coupling reaction between lithium diethylcopper and cis-1-chloro-1-octene.

Unlock Deck
Unlock for access to all 31 flashcards in this deck.
Unlock Deck
k this deck
25
Which of the following is a valid comparison of organocopper and organopalladium reagents?
A)Both work equally well with alkyl compounds.
B)Palladium compounds are more toxic than copper compounds.
C)Organopalladium reactions require a larger amount of the metal.
D)Organopalladium compounds are particularly useful in from biaryl compounds.
A)Both work equally well with alkyl compounds.
B)Palladium compounds are more toxic than copper compounds.
C)Organopalladium reactions require a larger amount of the metal.
D)Organopalladium compounds are particularly useful in from biaryl compounds.

Unlock Deck
Unlock for access to all 31 flashcards in this deck.
Unlock Deck
k this deck
26
Draw the condensed formula for the product formed when the following substance is treated with NBS using carbon tetrachloride as the solvent. 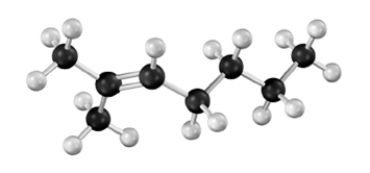


Unlock Deck
Unlock for access to all 31 flashcards in this deck.
Unlock Deck
k this deck
27
The following product is a simplified penicillin analog.Draw the structure of the missing reactant represented by the "?" when the reaction is carried out in the presence of a palladium catalyst. 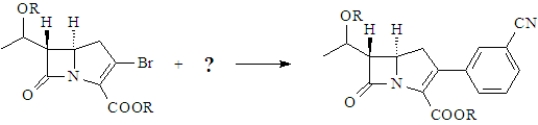
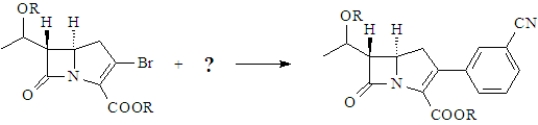

Unlock Deck
Unlock for access to all 31 flashcards in this deck.
Unlock Deck
k this deck
28
Consider the following reaction.  This reaction is classified as:
This reaction is classified as:
A)an oxidation.
B)a reduction.
C)neither an oxidation nor a reduction.
 This reaction is classified as:
This reaction is classified as:A)an oxidation.
B)a reduction.
C)neither an oxidation nor a reduction.

Unlock Deck
Unlock for access to all 31 flashcards in this deck.
Unlock Deck
k this deck
29
Draw the mechanism of production of trans-3-decene from trans-1-fluoro-1-heptene.

Unlock Deck
Unlock for access to all 31 flashcards in this deck.
Unlock Deck
k this deck
30
Which of the following is the least likely to form a Grignard reagent?
A)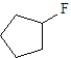
B)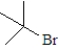
C)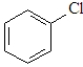
D)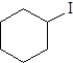
E)All will form Grignard reagents.
A)

B)

C)

D)

E)All will form Grignard reagents.

Unlock Deck
Unlock for access to all 31 flashcards in this deck.
Unlock Deck
k this deck
31
Which of the following pairs are not alternative reagents to prepare the indicated products?
A)HCl,SOCl2 - preparation of alkyl chlorides from alcohols
B)HBr,NBS - preparation of alkyl bromides from alkenes
C)Br2 and HBr - preparation of alkyl bromides from alkanes
D)diorganocopper reagents and organopalladium compounds - forming new carbon-to-carbon bonds
A)HCl,SOCl2 - preparation of alkyl chlorides from alcohols
B)HBr,NBS - preparation of alkyl bromides from alkenes
C)Br2 and HBr - preparation of alkyl bromides from alkanes
D)diorganocopper reagents and organopalladium compounds - forming new carbon-to-carbon bonds

Unlock Deck
Unlock for access to all 31 flashcards in this deck.
Unlock Deck
k this deck



