Deck 4: Organic Compounds: Cycloalkanes and Their Stereochemistry
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/37
Play
Full screen (f)
Deck 4: Organic Compounds: Cycloalkanes and Their Stereochemistry
1
Circle all bridgehead carbons in the following structure. 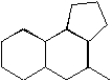


2
Exhibit 4-2
For each substituted cyclohexane below,draw its ring-flip isomer.Circle the most stable conformation.
Refer to Exhibit 4-2.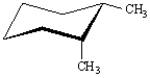
For each substituted cyclohexane below,draw its ring-flip isomer.Circle the most stable conformation.
Refer to Exhibit 4-2.


3
Exhibit 4-1
Refer to the structure below to answer the following question(s):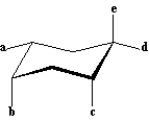
Refer to Exhibit 4-1.Which of the labeled bonds are trans to bond b?
Refer to the structure below to answer the following question(s):

Refer to Exhibit 4-1.Which of the labeled bonds are trans to bond b?
e
4
Draw 7 constitutional isomers of a cycloalkane with the formula C6H12.Name each of the isomers you drew.

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
5
Draw: cis-1-sec-butyl-2-ethylcyclopentane

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
6
Draw: 3-cyclobutylpentane

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
7
This question was omitted on the printed copy.This placeholder question is here to maintain the numbering system integrity between the printed copy and ExamView.Therefore,it has been marked "do not use on test" in ExamView's question information dialog.As a result,this placeholder question is automatically prevented from being chosen as a test question.

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
8
The energy difference of 3.8 kJ/mol between gauche and anti butane corresponds to an equilibrium constant,Keq,of approximately 1.9.Calculate the percentage of each conformer at equilibrium.

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
9
Exhibit 4-1
Refer to the structure below to answer the following question(s):
Refer to Exhibit 4-1.Which of the labeled bonds in the structure are equatorial bonds?
Refer to the structure below to answer the following question(s):

Refer to Exhibit 4-1.Which of the labeled bonds in the structure are equatorial bonds?

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
10
Draw: 1-chloro-2-isopropylcyclopentane

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
11
Below are the two chair conformations of a 1,2,4-trimethylcyclohexane.Estimate the amount of 1,3-diaxial strain in each conformer and predict which conformer is most stable. 


Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
12
Draw: 3,5-dicyclohexylnonane

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
13
Exhibit 4-1
Refer to the structure below to answer the following question(s):
Refer to Exhibit 4-1.Which labeled bonds have a 1,3-diaxial interaction with each other?
Refer to the structure below to answer the following question(s):

Refer to Exhibit 4-1.Which labeled bonds have a 1,3-diaxial interaction with each other?

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
14
Draw the two stereoisomers of 1,3-dibromocyclobutane.

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
15
Exhibit 4-3
The following question(s) refer to the structure of camphor shown below.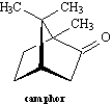
Refer to Exhibit 4-3.Circle all bridgehead carbons in camphor.
The following question(s) refer to the structure of camphor shown below.

Refer to Exhibit 4-3.Circle all bridgehead carbons in camphor.

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
16
Name: 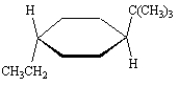


Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
17
The original question was combined with #1.This placeholder question is here to maintain the integrity of the numbering system between the printed copy and ExamView.Therefore,it has been marked "do not use on test" in ExamView's question information dialog.As a result,this placeholder question is automatically prevented from being chosen as a test question.

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
18
Exhibit 4-3
The following question(s) refer to the structure of camphor shown below.
Refer to Exhibit 4-3.Camphor is an example of a:
A)fused bicyclic molecule.
B)bridged bicyclic molecule.
C)fused tricyclic molecule.
D)bridged tricyclic molecule.
The following question(s) refer to the structure of camphor shown below.

Refer to Exhibit 4-3.Camphor is an example of a:
A)fused bicyclic molecule.
B)bridged bicyclic molecule.
C)fused tricyclic molecule.
D)bridged tricyclic molecule.

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
19
Exhibit 4-2
For each substituted cyclohexane below,draw its ring-flip isomer.Circle the most stable conformation.
Refer to Exhibit 4-2.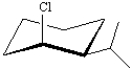
For each substituted cyclohexane below,draw its ring-flip isomer.Circle the most stable conformation.
Refer to Exhibit 4-2.


Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
20
Exhibit 4-2
For each substituted cyclohexane below,draw its ring-flip isomer.Circle the most stable conformation.
Refer to Exhibit 4-2.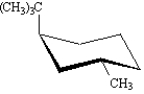
For each substituted cyclohexane below,draw its ring-flip isomer.Circle the most stable conformation.
Refer to Exhibit 4-2.


Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
21
Exhibit 4-4
Label each pair of compounds below as:
_____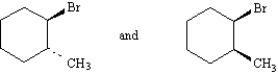
A)conformational isomers
B)stereoisomers
C)constitutional isomers
D)identical
Label each pair of compounds below as:
_____
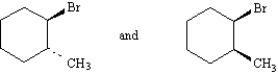
A)conformational isomers
B)stereoisomers
C)constitutional isomers
D)identical

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
22
(-)-Menthol can be isolated from the peppermint plant and is responsible for the characteristic flavor and taste of peppermint.The structure of (-)-menthol is:  On the chair template provided below,draw the two chair conformations that are in equilibrium for (-)-menthol.
On the chair template provided below,draw the two chair conformations that are in equilibrium for (-)-menthol. 
 On the chair template provided below,draw the two chair conformations that are in equilibrium for (-)-menthol.
On the chair template provided below,draw the two chair conformations that are in equilibrium for (-)-menthol. 

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
23
Which of the following would have the smallest strain energy (kJ/mol)?
A)cyclobutane
B)cyclopentane
C)cyclohexane
D)cyclooctane
A)cyclobutane
B)cyclopentane
C)cyclohexane
D)cyclooctane

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
24
In cyclopropane,which of the following strain types would be the least important in determining the overall energy?
A)angle
B)torsional
C)steric
D)All make a significant contribution.
A)angle
B)torsional
C)steric
D)All make a significant contribution.

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
25
In general,5-alkyl substituents in 1,3-dioxane exhibit a smaller equatorial preference than they do in cyclohexane.To what might you attribute this observation? 


Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
26
Consider the following table. Number of Carbon Atoms in Ring
Heat of Combustion per CH2 (kJ)
3
696
4
686
5
664
6
659
What is the approximate strain energy per CH2 for cyclopropane?
A)12 kJ
B)37 kJ
C)110 kJ
D)230
Heat of Combustion per CH2 (kJ)
3
696
4
686
5
664
6
659
What is the approximate strain energy per CH2 for cyclopropane?
A)12 kJ
B)37 kJ
C)110 kJ
D)230

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
27
D-Pinitol is an interesting hexahydroxy cyclohexane,whose structure is shown below. 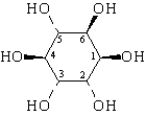 On the templates provided,draw the two chair conformations that are in equilibrium for D-pinitol.Circle the most stable conformation.
On the templates provided,draw the two chair conformations that are in equilibrium for D-pinitol.Circle the most stable conformation. 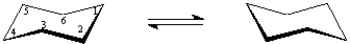
 On the templates provided,draw the two chair conformations that are in equilibrium for D-pinitol.Circle the most stable conformation.
On the templates provided,draw the two chair conformations that are in equilibrium for D-pinitol.Circle the most stable conformation. 

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
28
In methylcyclohexane:
A)all carbon atoms are sp3 hybridized.
B)ring-carbon atoms are sp2 hybridized and the methyl group is sp3 hybridized.
C)all bond angles are approximately 120°.
D)ring-bond angles are approximately 120° and the ring-methyl bond angle is approximately 109°.
A)all carbon atoms are sp3 hybridized.
B)ring-carbon atoms are sp2 hybridized and the methyl group is sp3 hybridized.
C)all bond angles are approximately 120°.
D)ring-bond angles are approximately 120° and the ring-methyl bond angle is approximately 109°.

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
29
Consider the two methyl groups indicated with letters in the following molecular model. 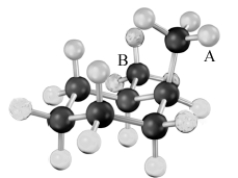 These two groups are:
These two groups are:
A)A: axial B: axial
B)A: axial B: equatorial
C)A: equatorial B: axial
D)A: equatorial B: equatorial
 These two groups are:
These two groups are:A)A: axial B: axial
B)A: axial B: equatorial
C)A: equatorial B: axial
D)A: equatorial B: equatorial

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
30
Which of the following would produce the greatest amount of 1,3-diaxial strain when substituted for Cl in the following structure? 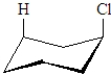
A)CN
B)OH
C)C(CH3)3
D)CO2H

A)CN
B)OH
C)C(CH3)3
D)CO2H

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
31
Consider the following table. Number of Carbon Atoms in Ring
Heat of Combustion per CH2 (kJ)
3
696
4
686
5
664
6
659
Based on the data in the table,which of the following compounds would have the largest strain energy?
A)cyclopropane
B)cyclobutane
C)cyclopentane
D)cyclohexane
Heat of Combustion per CH2 (kJ)
3
696
4
686
5
664
6
659
Based on the data in the table,which of the following compounds would have the largest strain energy?
A)cyclopropane
B)cyclobutane
C)cyclopentane
D)cyclohexane

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
32
What relationship exists between the following two structures? 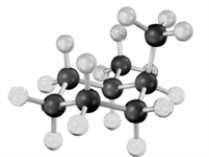
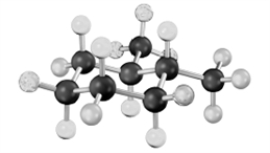
A)identical molecules
B)constitutional isomers
C)stereoisomers
D)different molecules


A)identical molecules
B)constitutional isomers
C)stereoisomers
D)different molecules

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
33
The two structures show below represent: 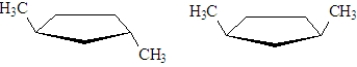
A)constitutional isomers
B)stereoisomers
C)cis-trans isomers
D)both b and c
E)a,b and c
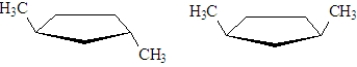
A)constitutional isomers
B)stereoisomers
C)cis-trans isomers
D)both b and c
E)a,b and c

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
34
Exhibit 4-4
Label each pair of compounds below as:
_____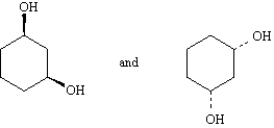
A)conformational isomers
B)stereoisomers
C)constitutional isomers
D)identical
Label each pair of compounds below as:
_____

A)conformational isomers
B)stereoisomers
C)constitutional isomers
D)identical

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
35
Exhibit 4-4
Label each pair of compounds below as:
_____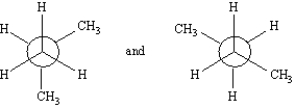
A)conformational isomers
B)stereoisomers
C)constitutional isomers
D)identical
Label each pair of compounds below as:
_____
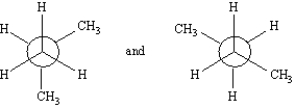
A)conformational isomers
B)stereoisomers
C)constitutional isomers
D)identical

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
36
Exhibit 4-4
Label each pair of compounds below as:
_____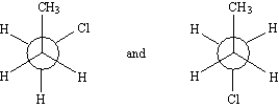
A)conformational isomers
B)stereoisomers
C)constitutional isomers
D)identical
Label each pair of compounds below as:
_____
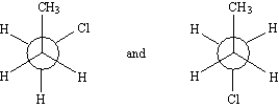
A)conformational isomers
B)stereoisomers
C)constitutional isomers
D)identical

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
37
Exhibit 4-4
Label each pair of compounds below as:
_____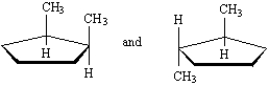
A)conformational isomers
B)stereoisomers
C)constitutional isomers
D)identical
Label each pair of compounds below as:
_____

A)conformational isomers
B)stereoisomers
C)constitutional isomers
D)identical

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck



