Deck 24: Amines and Heterocycles
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/41
Play
Full screen (f)
Deck 24: Amines and Heterocycles
1
Exhibit 24-4
Refer to the reaction below to answer the following question(s):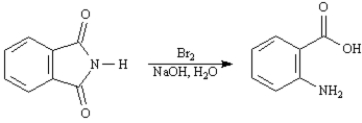
Refer to Exhibit 24-4.This reaction is an example of:
A)a Curtius rearrangement
B)a Hofmann elimination reaction
C)a Gabriel synthesis
D)a Hofmann rearrangement
Refer to the reaction below to answer the following question(s):

Refer to Exhibit 24-4.This reaction is an example of:
A)a Curtius rearrangement
B)a Hofmann elimination reaction
C)a Gabriel synthesis
D)a Hofmann rearrangement
a Hofmann rearrangement
2
Exhibit 24-8
Refer to the data below to answer the following question(s):
Triclocarban is a disinfectant prepared from 3,4-dichloroaniline and 4-chlorophenylisocyanate.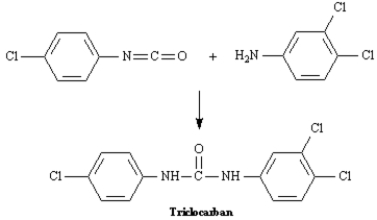
Refer to Exhibit 24-8.Propose a mechanism for the reaction of 3,4-dichloroaniline and 4-chlorophenylisocyanate to yield Triclocarban.
Refer to the data below to answer the following question(s):
Triclocarban is a disinfectant prepared from 3,4-dichloroaniline and 4-chlorophenylisocyanate.

Refer to Exhibit 24-8.Propose a mechanism for the reaction of 3,4-dichloroaniline and 4-chlorophenylisocyanate to yield Triclocarban.
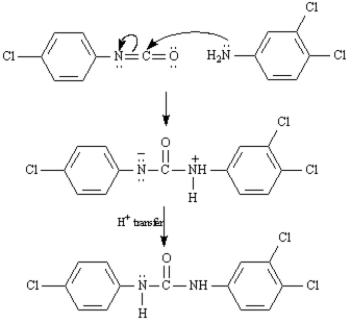
3
Name: 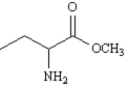

methyl 2-aminobutanoate
4
Name: 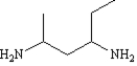


Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
5
Exhibit 24-3
Refer to the Table of pKas below to answer the following question(s):
pKas of Some Arylammonium Ions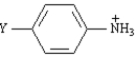 Y
Y
pKa
−H
4.63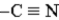 1.74
1.74
−NH2
6.15
Refer to Exhibit 24-3.Explain the difference in acidity between p-aminoanilinium ion and anilinium ion.Use both words and structures.
Refer to the Table of pKas below to answer the following question(s):
pKas of Some Arylammonium Ions
 Y
YpKa
−H
4.63
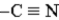 1.74
1.74−NH2
6.15
Refer to Exhibit 24-3.Explain the difference in acidity between p-aminoanilinium ion and anilinium ion.Use both words and structures.

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
6
Exhibit 24-8
Refer to the data below to answer the following question(s):
Triclocarban is a disinfectant prepared from 3,4-dichloroaniline and 4-chlorophenylisocyanate.
Refer to Exhibit 24-8.Prepare 4-chlorophenylisocyanate from toluene.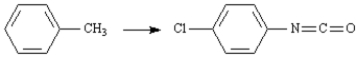
Refer to the data below to answer the following question(s):
Triclocarban is a disinfectant prepared from 3,4-dichloroaniline and 4-chlorophenylisocyanate.

Refer to Exhibit 24-8.Prepare 4-chlorophenylisocyanate from toluene.


Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
7
Name: 


Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
8
Exhibit 24-2
Consider the reaction below to answer the following question(s).
Methamphetamine can be synthesized by reacting phenyl-2-propanone with methylamine in the presence of H2/Ni.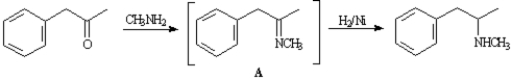
Refer to Exhibit 24-2.Intermediate A is an example of:
A)an imine
B)an enamine
C)an iminium ion
D)an imide
Consider the reaction below to answer the following question(s).
Methamphetamine can be synthesized by reacting phenyl-2-propanone with methylamine in the presence of H2/Ni.

Refer to Exhibit 24-2.Intermediate A is an example of:
A)an imine
B)an enamine
C)an iminium ion
D)an imide

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
9
Exhibit 24-3
Refer to the Table of pKas below to answer the following question(s):
pKas of Some Arylammonium Ions Y
Y
pKa
−H
4.63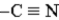 1.74
1.74
−NH2
6.15
Refer to Exhibit 24-3.Explain the difference in acidity between p-cyanoanilinium ion and anilinium ion.Use both words and structures.
Refer to the Table of pKas below to answer the following question(s):
pKas of Some Arylammonium Ions
 Y
YpKa
−H
4.63
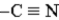 1.74
1.74−NH2
6.15
Refer to Exhibit 24-3.Explain the difference in acidity between p-cyanoanilinium ion and anilinium ion.Use both words and structures.

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
10
Exhibit 24-2
Consider the reaction below to answer the following question(s).
Methamphetamine can be synthesized by reacting phenyl-2-propanone with methylamine in the presence of H2/Ni.
Refer to Exhibit 24-2.Identify the nucleophile in the initial reaction of phenyl-2-propanone to yield intermediate A.
Consider the reaction below to answer the following question(s).
Methamphetamine can be synthesized by reacting phenyl-2-propanone with methylamine in the presence of H2/Ni.

Refer to Exhibit 24-2.Identify the nucleophile in the initial reaction of phenyl-2-propanone to yield intermediate A.

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
11
Name: 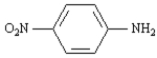


Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
12
Name:
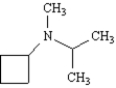


Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
13
Exhibit 24-3
Refer to the Table of pKas below to answer the following question(s):
pKas of Some Arylammonium Ions Y
Y
pKa
−H
4.63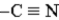 1.74
1.74
−NH2
6.15
Refer to Exhibit 24-3.Based on the pKas for their corresponding ammonium ions,which arylamine above is the strongest base?
Refer to the Table of pKas below to answer the following question(s):
pKas of Some Arylammonium Ions
 Y
YpKa
−H
4.63
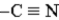 1.74
1.74−NH2
6.15
Refer to Exhibit 24-3.Based on the pKas for their corresponding ammonium ions,which arylamine above is the strongest base?

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
14
Exhibit 24-8
Refer to the data below to answer the following question(s):
Triclocarban is a disinfectant prepared from 3,4-dichloroaniline and 4-chlorophenylisocyanate.
Refer to Exhibit 24-8.Prepare 3,4-dichloroaniline from benzene.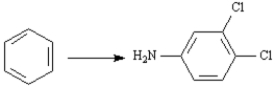
Refer to the data below to answer the following question(s):
Triclocarban is a disinfectant prepared from 3,4-dichloroaniline and 4-chlorophenylisocyanate.

Refer to Exhibit 24-8.Prepare 3,4-dichloroaniline from benzene.


Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
15
Exhibit 24-2
Consider the reaction below to answer the following question(s).
Methamphetamine can be synthesized by reacting phenyl-2-propanone with methylamine in the presence of H2/Ni.
Refer to Exhibit 24-2.Although the yield of methamphetamine is good,some unreacted phenyl-2-propanone remains after the reaction is complete.Describe how methamphetamine can be separated from phenyl-2-propanone.
Consider the reaction below to answer the following question(s).
Methamphetamine can be synthesized by reacting phenyl-2-propanone with methylamine in the presence of H2/Ni.

Refer to Exhibit 24-2.Although the yield of methamphetamine is good,some unreacted phenyl-2-propanone remains after the reaction is complete.Describe how methamphetamine can be separated from phenyl-2-propanone.

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
16
Rank the following compounds in order of increasing basicity.Label the least basic compound "1" and the most basic compound "4".Place the number corresponding to the compound's rank in the blank below the compound. 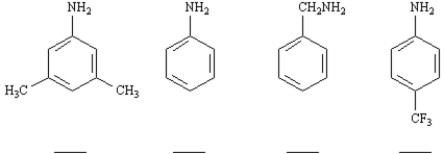


Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
17
Name: 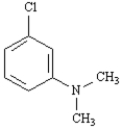


Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
18
NaOH,H2O
f.HNO3,H2SO4
f.HNO3,H2SO4


Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
19
When a THF solution of phenyl-2-propanone and bromobutane is treated with 50% aqueous NaOH,poor yields of 3-phenyl-2-heptanone result.However,when a small amount of benzyltriethylammonium chloride is added to the reaction mixture,the yield of 3-phenyl-2-heptanone increases to 90%.Explain these results. 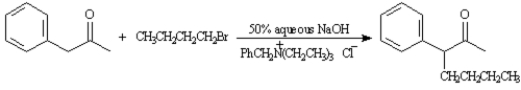
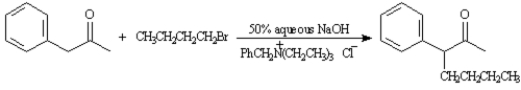

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
20
Exhibit 24-4
Refer to the reaction below to answer the following question(s):
Refer to Exhibit 24-4.Draw arrows on the structures provided below which show electron flow in the complete stepwise mechanism for the reaction above.
Refer to the reaction below to answer the following question(s):

Refer to Exhibit 24-4.Draw arrows on the structures provided below which show electron flow in the complete stepwise mechanism for the reaction above.


Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
21
Which of the following methods of preparation of amines can be used to prepare primary,secondary,and tertiary amines?
A)reduction of a nitrile
B)Gabriel synthesis from an alkyl halide
C)reduction amination of a ketone
D)Hofman rearrangement of an amide
E)None of these methods is applicable to all types of amines.
A)reduction of a nitrile
B)Gabriel synthesis from an alkyl halide
C)reduction amination of a ketone
D)Hofman rearrangement of an amide
E)None of these methods is applicable to all types of amines.

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
22
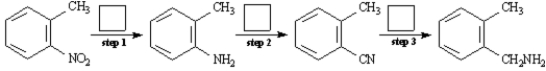
Draw the structures for each of the intermediates in the boxes provided for the synthesis below. 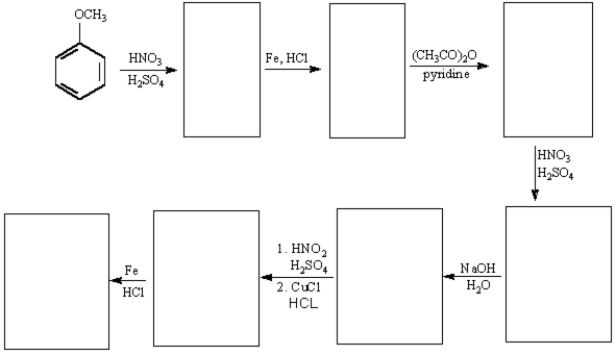


Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
23
Describe the importance of the formation of arenediazonium salts.

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
24
Which of the following would best represent the form of ethylamine (pKa) at physiological pH?
A)CH3CH2NH3+
B)CH3CH2NH2
C)CH3CH2NH-
D)a combination of a and b
A)CH3CH2NH3+
B)CH3CH2NH2
C)CH3CH2NH-
D)a combination of a and b

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
25
An unknown substance produced the following spectrum. 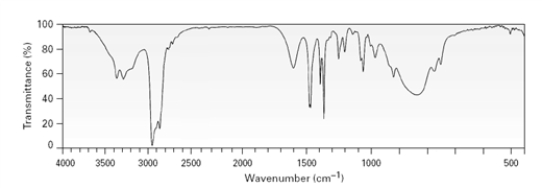 This unknown is likely to be a:
This unknown is likely to be a:
A)primary amine
B)secondary amine
C)tertiary amine
D)amide
 This unknown is likely to be a:
This unknown is likely to be a:A)primary amine
B)secondary amine
C)tertiary amine
D)amide

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
26
Name the following substance.Atoms other than carbon and hydrogen are labeled. 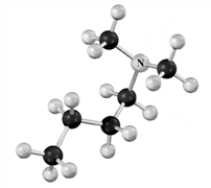


Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
27
Classify the following amine.Atoms other than carbon and hydrogen are labeled. 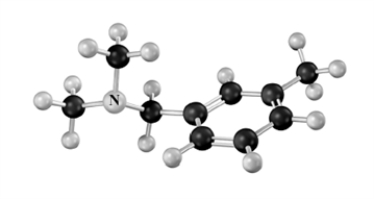
A)primary
B)secondary
C)tertiary
D)quaternary

A)primary
B)secondary
C)tertiary
D)quaternary

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
28
Write the equation for the reaction represented by the pKa of methyl amine.

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
29
Propose a structure that is consistent with the following spectral data:
MS:
M+ at 73
IR:
3350 cm−1 (weak,single band)
1H NMR:
δ1.05 (br s,1H),δ1.15 (t,6H),δ2.65 (q,4H)
MS:
M+ at 73
IR:
3350 cm−1 (weak,single band)
1H NMR:
δ1.05 (br s,1H),δ1.15 (t,6H),δ2.65 (q,4H)

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
30
Draw the mechanism for the Hofmann rearrangement of acetamide to acetamine.

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
31
At which pH will a solution of a 0.00050 M solution of aniline (pKa = 4.63) contain the greatest amount of the neutral species?
A)2.74
B)4.63
C)7.31
D)9.90
A)2.74
B)4.63
C)7.31
D)9.90

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
32
Which of the following methods of preparation of amines results in an amine with one less carbon atom than the starting material?
A)reduction of a nitrile
B)Gabriel synthesis from an alkyl halide
C)reductive amination of a ketone
D)Hofman rearrangement of an amide
A)reduction of a nitrile
B)Gabriel synthesis from an alkyl halide
C)reductive amination of a ketone
D)Hofman rearrangement of an amide

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
33
Draw the product of the reaction of the following substance with 1) LiAlH4 in ether,2) H2O.From what alkyl bromide could this substance be produced when reacted with NaCN? Atoms other than carbon and hydrogen are labeled. 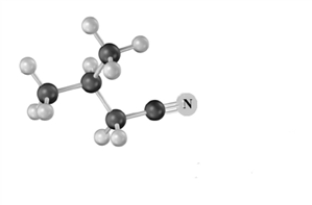


Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
34
Which of the following is not classified as a heterocycle?
A)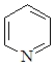
B)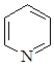
C)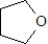
D)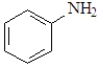
E)c and d
A)

B)

C)

D)

E)c and d

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
35
Draw the mechanism of the reductive amination of 2-butanone in the presence of ammonia and hydrochloric acid.

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
36
Compared to pentylamine,pentane is
A)more soluble in water.
B)has a higher boiling point.
C)is less basic.
D)more offensive in terms of odor.
A)more soluble in water.
B)has a higher boiling point.
C)is less basic.
D)more offensive in terms of odor.

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
37
What is the percentage of neutral species in a 0.00500 M solution of pyridine at pH 9.50? pKa of pyridine is 5.25.
A)25%
B)75%
C)50%
D)nearly 100%
E)close to 0%
A)25%
B)75%
C)50%
D)nearly 100%
E)close to 0%

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
38
The pKa of diethylammonium ion is 10.98.What is the pKb of diethylamine?
A)3.02
B)14.00
C)24.98
D)7.00
A)3.02
B)14.00
C)24.98
D)7.00

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
39
Which of the following is not a valid comparison of the spectral data for alkyl amines and alkyl alcohols?
A)IR: strong absorptions > 3000 cm-1.
B)MS: peaks due α-cleavage
C)(13C NMR: adjacent carbon signal shifted downfield relative to an alkane)
D)(1H NMR: proton bonded to the heteroatom appears over a wide range)
E)All are valid comparisons between the spectral data of amines and alcohols.
A)IR: strong absorptions > 3000 cm-1.
B)MS: peaks due α-cleavage
C)(13C NMR: adjacent carbon signal shifted downfield relative to an alkane)
D)(1H NMR: proton bonded to the heteroatom appears over a wide range)
E)All are valid comparisons between the spectral data of amines and alcohols.

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
40
The amino acid phenylalanine has the structure below. 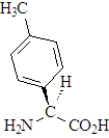 Which of the following is the best representation of this acid at pH 7.3?
Which of the following is the best representation of this acid at pH 7.3?
A)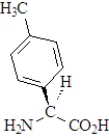
B)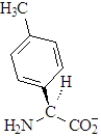
C)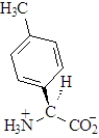
D)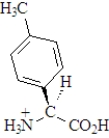
 Which of the following is the best representation of this acid at pH 7.3?
Which of the following is the best representation of this acid at pH 7.3?A)

B)

C)

D)
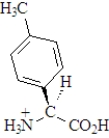

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
41
Draw the mechanism of the Sandmeyer reaction that converts o-methyl aniline to o-bromotoluene.

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck



