
Essentials of the Living World 5th Edition by George Johnson
Edition 5ISBN: 978-0078096945
Essentials of the Living World 5th Edition by George Johnson
Edition 5ISBN: 978-0078096945 Exercise 2
Using Radioactive Decay to Date the Iceman
In the fall of 1991, sticking out of the melting snow on the crest of a high pass near the mountainous border between Italy and Austria, two German hikers found a corpse. Right away it was clear the body was very old, frozen in an icy trench where he had sought shelter long ago and only now released as the ice melted. In the years since this startling find, scientists have learned a great deal about the dead man, who they named Ötzi. They know his age, his health, the clothing he wore, what he ate, and that he died from an arrow that ripped through his back. Its tip is still embedded in the back of his left shoulder. From the distribution of chemicals in his teeth and bones, we know he lived his life within 60 kilometers of where he died.
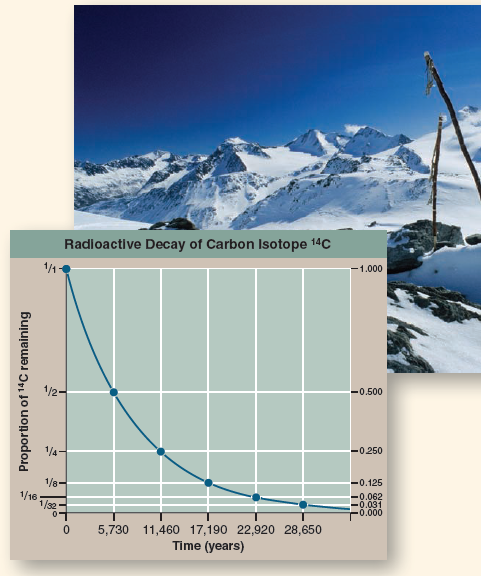
When did this Iceman die? Scientists answered this key question by measuring the degree of decay of the short-lived carbon isotope 14 C in Ötzi's body. While most carbon atoms are the stable isotope 12 C, a tiny proportion are the unstable radioactive isotope 14 C, created by the bombardment of nitrogen-14 ( 14 N) atoms with cosmic rays. This proportion of 14 C is captured by plants in photosynthesis and is present in the carbon molecules of the animal's body that eats the plant. After the plant or animal dies, it no longer accumulates any more carbon, and the 14 C present at the time of death decays over time back to 14 N. Thus, over time the ratio of 14 C to 12 C decreases. It takes 5,730 years for half of the 14 C present to decay, a length of time called the half-life of the 14 C isotope. Because the half-life is a constant that never changes, the extent of radioactive decay allows you to date a sample. Thus a sample that had one-quarter of its original proportion of 14 C remaining would be approximately 11,460 years old (two half-lives).
The graph to the right displays the radioactive decay curve of the carbon isotope 14 C. Scientists know it takes 5,730 years for half of the 14 C present in a sample to decay to nitrogen-14 ( 14 N). When Ötzi's carbon isotopes were analyzed, researchers determined that the ratio of 14 C to 12 C (a ratio is the size of one variable relative to another), also written as the fraction 14 C/ 12 C, in Ötzi's body was 0.435 of the fraction found in tissues of a person who has recently died.
Making Inferences If Ötzi were indeed a recent corpse, made to look old by the harsh weather conditions found on the high mountain pass, what would you expect the ratio of 14 C to 12 C to be, relative to that in your own body?
In the fall of 1991, sticking out of the melting snow on the crest of a high pass near the mountainous border between Italy and Austria, two German hikers found a corpse. Right away it was clear the body was very old, frozen in an icy trench where he had sought shelter long ago and only now released as the ice melted. In the years since this startling find, scientists have learned a great deal about the dead man, who they named Ötzi. They know his age, his health, the clothing he wore, what he ate, and that he died from an arrow that ripped through his back. Its tip is still embedded in the back of his left shoulder. From the distribution of chemicals in his teeth and bones, we know he lived his life within 60 kilometers of where he died.
When did this Iceman die? Scientists answered this key question by measuring the degree of decay of the short-lived carbon isotope 14 C in Ötzi's body. While most carbon atoms are the stable isotope 12 C, a tiny proportion are the unstable radioactive isotope 14 C, created by the bombardment of nitrogen-14 ( 14 N) atoms with cosmic rays. This proportion of 14 C is captured by plants in photosynthesis and is present in the carbon molecules of the animal's body that eats the plant. After the plant or animal dies, it no longer accumulates any more carbon, and the 14 C present at the time of death decays over time back to 14 N. Thus, over time the ratio of 14 C to 12 C decreases. It takes 5,730 years for half of the 14 C present to decay, a length of time called the half-life of the 14 C isotope. Because the half-life is a constant that never changes, the extent of radioactive decay allows you to date a sample. Thus a sample that had one-quarter of its original proportion of 14 C remaining would be approximately 11,460 years old (two half-lives).
The graph to the right displays the radioactive decay curve of the carbon isotope 14 C. Scientists know it takes 5,730 years for half of the 14 C present in a sample to decay to nitrogen-14 ( 14 N). When Ötzi's carbon isotopes were analyzed, researchers determined that the ratio of 14 C to 12 C (a ratio is the size of one variable relative to another), also written as the fraction 14 C/ 12 C, in Ötzi's body was 0.435 of the fraction found in tissues of a person who has recently died.


Making Inferences If Ötzi were indeed a recent corpse, made to look old by the harsh weather conditions found on the high mountain pass, what would you expect the ratio of 14 C to 12 C to be, relative to that in your own body?
Explanation
If Otzi were indeed a recent corpse, mad...
Essentials of the Living World 5th Edition by George Johnson
Why don’t you like this exercise?
Other Minimum 8 character and maximum 255 character
Character 255



