
College Physics 10th Edition by Raymond Serway,Chris Vuille
Edition 10ISBN: 978-1285737041
College Physics 10th Edition by Raymond Serway,Chris Vuille
Edition 10ISBN: 978-1285737041 Exercise 32
Assume ordinary soil contains natural uranium in amounts of 1 part per million by mass. (a) How much uranium is in the top 1.00 m of soil on a 1-acre (43 560-ft 2 ) plot of ground, assuming the specific gravity of soil is 4.00 (b) How much of the isotope 235 U, appropriate for nuclear reactor fuel, is in this soil Hint: See Appendix B for the percent abundance of 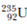 .
.
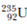 .
.Explanation
(a)
Find the mass of soil in a given par...
College Physics 10th Edition by Raymond Serway,Chris Vuille
Why don’t you like this exercise?
Other Minimum 8 character and maximum 255 character
Character 255



