
Biomedical Engineering: Bridging Medicine and Technology 1st Edition by Veronique Tran,Mark Saltzman
Edition 1ISBN: 9780521840996
Biomedical Engineering: Bridging Medicine and Technology 1st Edition by Veronique Tran,Mark Saltzman
Edition 1ISBN: 9780521840996 Exercise 21
Identify the acid and conjugate base in each reaction. Calculate the pKa for each acid. List them in order from the strongest to weakest acid. The acid-ionization constants, Ka,at25◦C are listed for each.
a.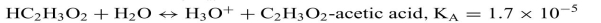
b.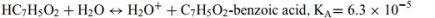
c.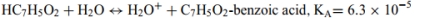
d.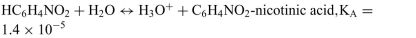
a.
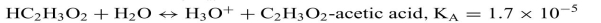
b.
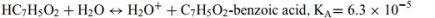
c.
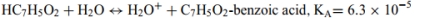
d.
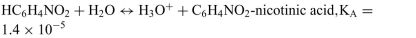
Explanation
a) The pKa for acetic acid is 0.000017 ...
Biomedical Engineering: Bridging Medicine and Technology 1st Edition by Veronique Tran,Mark Saltzman
Why don’t you like this exercise?
Other Minimum 8 character and maximum 255 character
Character 255



