Deck 4: Chemical Reactions
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/72
Play
Full screen (f)
Deck 4: Chemical Reactions
1
Which one of the statements below is false concerning the following reaction: NH3(g)+ H2O 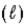
 NH4+(aq)+ OH−(aq)
NH4+(aq)+ OH−(aq)
A) The double arrows indicate that ammonia,NH3,is only very slightly soluble in water.
B) The reaction is reversible.
C) When NH3 is added to H2O,NH4+ and OH− ions are produced in a 1:1 ratio.
D) When solutions of NH4+ and OH- are mixed,some ammonia is produced.
E) Ammonia partially reacts with water.
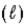
 NH4+(aq)+ OH−(aq)
NH4+(aq)+ OH−(aq)A) The double arrows indicate that ammonia,NH3,is only very slightly soluble in water.
B) The reaction is reversible.
C) When NH3 is added to H2O,NH4+ and OH− ions are produced in a 1:1 ratio.
D) When solutions of NH4+ and OH- are mixed,some ammonia is produced.
E) Ammonia partially reacts with water.
The double arrows indicate that ammonia,NH3,is only very slightly soluble in water.
2
What are the smallest whole number coefficients the reactants and products when the following molecular equation is correctly balanced? __NH3(g)+__O2(g)→ __NO(g)+ __H2O(g)
A) 1,1,1,1
B) 2,3,2,3
C) 3,2,3,4
D) 3,4,3,4
E) 4,5,4,6
A) 1,1,1,1
B) 2,3,2,3
C) 3,2,3,4
D) 3,4,3,4
E) 4,5,4,6
4,5,4,6
3
Which of the following reactions best describes the dissolution of solid CsOH(s)in water?
A) CsOH(s)+ H2O(l)→ CsO-(aq)+ H3O+(aq)
B) CsOH(s)→ CsO-(aq)+ H+(aq)
C) CsOH(s)→ Cs+(aq)+ OH-(aq)
D) CsOH(s)→ CsO+(aq)+ H-(aq)
E) CsOH(s)→ CsOH(aq)
A) CsOH(s)+ H2O(l)→ CsO-(aq)+ H3O+(aq)
B) CsOH(s)→ CsO-(aq)+ H+(aq)
C) CsOH(s)→ Cs+(aq)+ OH-(aq)
D) CsOH(s)→ CsO+(aq)+ H-(aq)
E) CsOH(s)→ CsOH(aq)
CsOH(s)→ Cs+(aq)+ OH-(aq)
4
Which one of the following equations is properly balanced?
A) Sn + 4HNO3 → SnO2 + 4NO2 + 2H2O
B) 2Na2SO4 + 3Bi(NO3)3 → Bi2(SO4)3 + 9NaNO3
C) CH3CHO + 3O2 → 2CO2 + 2H2O
D) NH4NO3 → 2H2O + N2
E) Na2CO3 + 2H2SO4 → Na2SO4 + 2H2O + CO2
A) Sn + 4HNO3 → SnO2 + 4NO2 + 2H2O
B) 2Na2SO4 + 3Bi(NO3)3 → Bi2(SO4)3 + 9NaNO3
C) CH3CHO + 3O2 → 2CO2 + 2H2O
D) NH4NO3 → 2H2O + N2
E) Na2CO3 + 2H2SO4 → Na2SO4 + 2H2O + CO2

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
5
Which anion will form a precipitate with Ba2+?
A) Cl-
B) SO42-
C) C2H3O2-
D) Br-
E) None of these
A) Cl-
B) SO42-
C) C2H3O2-
D) Br-
E) None of these

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
6
What is the smallest whole number coefficient for sodium hydroxide when the following equation is balanced? Na2SO4 + Al(OH)3 → Al2(SO4)3 + NaOH
A) 1
B) 2
C) 3
D) 4
E) 6
A) 1
B) 2
C) 3
D) 4
E) 6

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
7
Which of the following compounds is insoluble in water?
A) NH4Br
B) KBr
C) FeCl2
D) Hg2Br2
E) LiBr
A) NH4Br
B) KBr
C) FeCl2
D) Hg2Br2
E) LiBr

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
8
Which of the following ions is most likely to form an insoluble compound when combined with sulfate ion?
A) Sr2+
B) I-
C) K+
D) Na+
E) S2-
A) Sr2+
B) I-
C) K+
D) Na+
E) S2-

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
9
A precipitate is expected when an aqueous solution of sodium iodide is added to an aqueous solution of
A) calcium nitrate.
B) barium hydroxide.
C) lead perchlorate.
D) iron(II)chloride.
E) sodium sulfate.
A) calcium nitrate.
B) barium hydroxide.
C) lead perchlorate.
D) iron(II)chloride.
E) sodium sulfate.

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
10
The products of the complete combustion of octane,C8H18,are carbon dioxide and water.Write a balanced chemical equation for this reaction.
A) C8H18(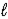 )→ 8 C(s)+ 9 H2(g)
)→ 8 C(s)+ 9 H2(g)
B) C8H18(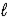 )+ 25 O2(g)→ 8 CO2(g)+ 9 H2O(g)
)+ 25 O2(g)→ 8 CO2(g)+ 9 H2O(g)
C) 2 C8H18(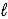 )+ 25 O2(g)→ 16 CO2(g)+ 18 H2O(g)
)+ 25 O2(g)→ 16 CO2(g)+ 18 H2O(g)
D) C8H18(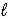 )+ 16 O2(g)→ 8 CO2(g)+ 9 H2(g)
)+ 16 O2(g)→ 8 CO2(g)+ 9 H2(g)
E) 2 C8H18(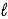 )+ 17 O2(g)→ 16 CO(g)+ 18 H2O(g)
)+ 17 O2(g)→ 16 CO(g)+ 18 H2O(g)
A) C8H18(
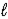 )→ 8 C(s)+ 9 H2(g)
)→ 8 C(s)+ 9 H2(g)B) C8H18(
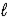 )+ 25 O2(g)→ 8 CO2(g)+ 9 H2O(g)
)+ 25 O2(g)→ 8 CO2(g)+ 9 H2O(g)C) 2 C8H18(
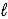 )+ 25 O2(g)→ 16 CO2(g)+ 18 H2O(g)
)+ 25 O2(g)→ 16 CO2(g)+ 18 H2O(g)D) C8H18(
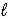 )+ 16 O2(g)→ 8 CO2(g)+ 9 H2(g)
)+ 16 O2(g)→ 8 CO2(g)+ 9 H2(g)E) 2 C8H18(
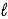 )+ 17 O2(g)→ 16 CO(g)+ 18 H2O(g)
)+ 17 O2(g)→ 16 CO(g)+ 18 H2O(g)
Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
11
Which of the following compounds is soluble in water?
A) Al2S3
B) Cs2O
C) CaS
D) Hg2Cl2
E) NiO
A) Al2S3
B) Cs2O
C) CaS
D) Hg2Cl2
E) NiO

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
12
A precipitate will form when aqueous nickel(II)chloride is added to an aqueous solution of ____.
A) SrI2
B) Cu(NO3)2
C) KOH
D) Na2SO4
E) NaBr
A) SrI2
B) Cu(NO3)2
C) KOH
D) Na2SO4
E) NaBr

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
13
Which anion will form a precipitate with K+?
A) Cl-
B) SO42-
C) C2H3O2-
D) S2-
E) none
A) Cl-
B) SO42-
C) C2H3O2-
D) S2-
E) none

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
14
All of the following compounds are insoluble in water except _____.
A) BaCO3
B) PbF2
C) Fe(OH)3
D) Ni(ClO4)2
E) PbCrO4
A) BaCO3
B) PbF2
C) Fe(OH)3
D) Ni(ClO4)2
E) PbCrO4

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
15
Which of the following equations is not balanced?
A) 2Sb2OS2 + 10O2 → 2Sb2O5 + 4SO3
B) (NH4)2Cr2O7 → N2 + 4H2O + Cr2O3
C) C12H22O11 + 12O2 → 12CO2 + 11H2O
D) 2NaCl + Pb(NO3)2 → PbCl2 + 2NaNO3
E) Fe3O4 + 3CO → 3Fe + 3CO2
A) 2Sb2OS2 + 10O2 → 2Sb2O5 + 4SO3
B) (NH4)2Cr2O7 → N2 + 4H2O + Cr2O3
C) C12H22O11 + 12O2 → 12CO2 + 11H2O
D) 2NaCl + Pb(NO3)2 → PbCl2 + 2NaNO3
E) Fe3O4 + 3CO → 3Fe + 3CO2

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
16
Metals react with oxygen gas to produce oxides with the general formula MxOy.Write a balanced chemical equation for the reaction of titanium with oxygen to yield titanium(IV)oxide.
A) 4 Ti(s)+ O2(g)→ 2 Ti2O(s)
B) Ti(s)+ O2(g)→ TiO2(s)
C) 2 Ti(s)+ O2(g)→ 2 TiO(s)
D) Ti(s)+ O(g)→ TiO(s)
E) 8 Ti(s)+ O2(g)→ 2 Ti4O(s)
A) 4 Ti(s)+ O2(g)→ 2 Ti2O(s)
B) Ti(s)+ O2(g)→ TiO2(s)
C) 2 Ti(s)+ O2(g)→ 2 TiO(s)
D) Ti(s)+ O(g)→ TiO(s)
E) 8 Ti(s)+ O2(g)→ 2 Ti4O(s)

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
17
A precipitate will form when aqueous Pb(NO3)2 is added to an aqueous solution of ____.
A) Cu(NO3)2
B) NaI
C) NaCH3CO2
D) Pb(ClO4)2
E) KNO3
A) Cu(NO3)2
B) NaI
C) NaCH3CO2
D) Pb(ClO4)2
E) KNO3

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
18
The reaction of chlorine molecule with potassium iodide yields iodine molecule and potassium chloride.Write a balanced chemical equation for this reaction.
A) Cl2(g)+ KI(s)→ I(s)+ KCl2(s)
B) Cl2(g)+ 2 KI(s)→ I2(s)+ 2 KCl(s)
C) Cl2(g)+ KI2(s)→ I2(s)+ KCl2(s)
D) Cl(g)+ KI(s)→ I(s)+ KCl(s)
E) Cl2(g)+ 2 K2I(s)→ I2(s)+ 2 K2Cl(s)
A) Cl2(g)+ KI(s)→ I(s)+ KCl2(s)
B) Cl2(g)+ 2 KI(s)→ I2(s)+ 2 KCl(s)
C) Cl2(g)+ KI2(s)→ I2(s)+ KCl2(s)
D) Cl(g)+ KI(s)→ I(s)+ KCl(s)
E) Cl2(g)+ 2 K2I(s)→ I2(s)+ 2 K2Cl(s)

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
19
All of the following compounds are soluble in water except ____.
A) CoCO3
B) Cs2CO3
C) (NH4)2CO3
D) K2CO3
E) Na2CO3
A) CoCO3
B) Cs2CO3
C) (NH4)2CO3
D) K2CO3
E) Na2CO3

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
20
Which of the following compounds is soluble in water?
A) Fe2O3
B) Rb2O
C) BaS
D) Hg2I2
E) ZnO
A) Fe2O3
B) Rb2O
C) BaS
D) Hg2I2
E) ZnO

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
21
Which of the following would not be depicted as the individual ions on the reactant side of a complete ionic reaction?
A) LiOH
B) HBr
C) SrCl2
D) CH3COOH
E) CoCl3
A) LiOH
B) HBr
C) SrCl2
D) CH3COOH
E) CoCl3

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
22
Which of the following compounds acts as a strong base in an aqueous solution?
A) HOCH2CH2OH
B) Sr(OH)2
C) H3PO4
D) NH3
E) HNO3
A) HOCH2CH2OH
B) Sr(OH)2
C) H3PO4
D) NH3
E) HNO3

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
23
If an aqueous solution of _____ is added to a mixture of Pb2+ and Ba2+,the lead ion will precipitate,but the barium ion will remain in solution.
A) NaOH
B) Na2SO4
C) K3PO4
D) KCO3
E) NaF
A) NaOH
B) Na2SO4
C) K3PO4
D) KCO3
E) NaF

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
24
Write a balanced equation for the reaction that occurs when hydrogen sulfate ion behaves as a Brønsted-Lowry acid in water.
A) HSO4-(aq)+ H2O(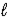 )
) 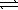 H2SO4(aq)+ H3O+(aq)
H2SO4(aq)+ H3O+(aq)
B) SO42-(aq)+ H2O(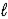 )
) 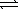 HSO4-(aq)+ OH-(aq)
HSO4-(aq)+ OH-(aq)
C) HSO4-(aq)+ H2O(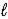 )
) 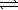 SO42-(aq)+ H3O+(aq)
SO42-(aq)+ H3O+(aq)
D) HSO4-(aq)+ H2O(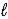 )
) 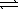 H2SO4(aq)+ OH-(aq)
H2SO4(aq)+ OH-(aq)
E) H2SO4(aq)+ H2O(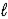 )
) 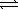 HSO4-(aq)+ H3O+(aq)
HSO4-(aq)+ H3O+(aq)
A) HSO4-(aq)+ H2O(
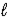 )
) 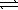 H2SO4(aq)+ H3O+(aq)
H2SO4(aq)+ H3O+(aq)B) SO42-(aq)+ H2O(
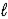 )
) 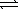 HSO4-(aq)+ OH-(aq)
HSO4-(aq)+ OH-(aq)C) HSO4-(aq)+ H2O(
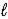 )
) 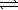 SO42-(aq)+ H3O+(aq)
SO42-(aq)+ H3O+(aq)D) HSO4-(aq)+ H2O(
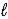 )
) 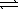 H2SO4(aq)+ OH-(aq)
H2SO4(aq)+ OH-(aq)E) H2SO4(aq)+ H2O(
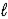 )
) 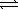 HSO4-(aq)+ H3O+(aq)
HSO4-(aq)+ H3O+(aq)
Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
25
Which of the following compounds is a weak acid in aqueous solution?
A) HCl
B) H3PO4
C) HNO3
D) HClO4
E) H2SO4
A) HCl
B) H3PO4
C) HNO3
D) HClO4
E) H2SO4

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
26
Write a balanced chemical equation for the reaction of aqueous solutions of sodium sulfide and zinc(II)chloride.
A) Na2S(aq)+ ZnCl2(aq)→ ZnS(s)+ 2 NaCl(aq)
B) Na2S(aq)+ ZnCl2(aq)→ ZnS(s)+ 2 NaCl(s)
C) Na2S(aq)+ ZnCl2(aq)→ Na2Zn(s)+ SCl2(aq)
D) Na2S(aq)+ ZnCl2(aq)→ Na2Zn(aq)+ SCl2(g)
E) None of these
A) Na2S(aq)+ ZnCl2(aq)→ ZnS(s)+ 2 NaCl(aq)
B) Na2S(aq)+ ZnCl2(aq)→ ZnS(s)+ 2 NaCl(s)
C) Na2S(aq)+ ZnCl2(aq)→ Na2Zn(s)+ SCl2(aq)
D) Na2S(aq)+ ZnCl2(aq)→ Na2Zn(aq)+ SCl2(g)
E) None of these

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
27
Which of the following compounds cannot be depicted as the individual ions on the reactant side in a complete ionic reaction?
A) CsOH
B) HCl
C) Ca(NO3)2
D) CaCO3
E) Ti(NO3)3
A) CsOH
B) HCl
C) Ca(NO3)2
D) CaCO3
E) Ti(NO3)3

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
28
Which of the following is a weak base in aqueous solution?
A) RbCl
B) KOH
C) NH3
D) HBr
E) Sr(OH)2
A) RbCl
B) KOH
C) NH3
D) HBr
E) Sr(OH)2

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
29
Which of the following products will form on mixing aqueous solutions of Cu(NO3)2 and NaOH?
A) Cu(OH)2(s),Na+(aq),and NO3−(aq)
B) Cu(OH)2(s)and NaNO3(s)
C) Cu2(OH)2(aq)and NaNO3(aq)
D) Cu(OH)2(aq)and NaNO3(s)
E) Cu(OH)2(s),N2(g),and H2O(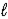 )
)
A) Cu(OH)2(s),Na+(aq),and NO3−(aq)
B) Cu(OH)2(s)and NaNO3(s)
C) Cu2(OH)2(aq)and NaNO3(aq)
D) Cu(OH)2(aq)and NaNO3(s)
E) Cu(OH)2(s),N2(g),and H2O(
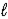 )
)
Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
30
When solutions of barium iodide and lithium sulfate are mixed,the spectator ions in the resulting precipitation reaction are
A) only SO42-.
B) both Li+ and I-.
C) only I-.
D) only Li+.
E) only Ba2+.
A) only SO42-.
B) both Li+ and I-.
C) only I-.
D) only Li+.
E) only Ba2+.

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
31
Nitric acid is the product of the reaction of ____ and H2O.
A) SO3
B) NO2
C) N2
D) NH3
E) N3-
A) SO3
B) NO2
C) N2
D) NH3
E) N3-

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
32
Which of the following equation best represents the net ionic equation for the reaction between aqueous solutions of barium chloride and cesium sulfate?
A) 2 H+(aq)+ 2 Cl-(aq)→ 2 HCl(g)
B) Ba2+(aq)+ SO42-(aq)→ BaSO4(s)
C) Ba2+(aq)+ 2 Cl-(aq)+ 2 Cs+(aq)+ SO42-(aq)→ BaSO4(s)+ 2 CsCl(aq)
D) BaCl2(aq)+ Cs2SO4(aq)→ BaSO4(s)+ 2 CsCl(aq)
E) None of these
A) 2 H+(aq)+ 2 Cl-(aq)→ 2 HCl(g)
B) Ba2+(aq)+ SO42-(aq)→ BaSO4(s)
C) Ba2+(aq)+ 2 Cl-(aq)+ 2 Cs+(aq)+ SO42-(aq)→ BaSO4(s)+ 2 CsCl(aq)
D) BaCl2(aq)+ Cs2SO4(aq)→ BaSO4(s)+ 2 CsCl(aq)
E) None of these

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
33
If an aqueous solution of ____ is added to a mixture of F- and SO42-,the fluoride ion will precipitate,but the sulfate ion will remain in solution.
A) LiBr
B) HNO3
C) Pb(ClO4)2
D) MgNO3
E) AlCl3
A) LiBr
B) HNO3
C) Pb(ClO4)2
D) MgNO3
E) AlCl3

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
34
Write a balanced chemical equation for the reaction of aqueous solutions of magnesium chloride and potassium phosphate.
A) MgCl2(aq)+ K3PO4(aq)→ K3Mg(s)+ PO4Cl2(aq)
B) 3 MgCl2(aq)+ 2 K3PO4(aq)→ 3 K2Mg(s)+ 2 PO4Cl3(aq)
C) MgCl(aq)+ KPO4(aq)→ MgPO4(s)+ KCl(aq)
D) MgCl2(aq)+ 2 KPO4(aq)→ Mg(PO4)2(s)+ 2 KCl(aq)
E) 3 MgCl2(aq)+ 2 K3PO4(aq)→ Mg3(PO4)2(s)+ 6 KCl(aq)
A) MgCl2(aq)+ K3PO4(aq)→ K3Mg(s)+ PO4Cl2(aq)
B) 3 MgCl2(aq)+ 2 K3PO4(aq)→ 3 K2Mg(s)+ 2 PO4Cl3(aq)
C) MgCl(aq)+ KPO4(aq)→ MgPO4(s)+ KCl(aq)
D) MgCl2(aq)+ 2 KPO4(aq)→ Mg(PO4)2(s)+ 2 KCl(aq)
E) 3 MgCl2(aq)+ 2 K3PO4(aq)→ Mg3(PO4)2(s)+ 6 KCl(aq)

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
35
What base results when K2O reacts with water?
A) H3O+(aq)
B) KH(aq)
C) O2-(aq)
D) KOH(aq)
E) KO-(aq)
A) H3O+(aq)
B) KH(aq)
C) O2-(aq)
D) KOH(aq)
E) KO-(aq)

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
36
Which of the following is a strong acid in aqueous solution?
A) HOCH2CH2OH
B) Ca(OH)2
C) H3PO4
D) NH3
E) HClO4
A) HOCH2CH2OH
B) Ca(OH)2
C) H3PO4
D) NH3
E) HClO4

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
37
When aqueous solutions of calcium iodide,CaI2,and silver nitrate,AgNO3,are combined,which of the following statements below describes what occurs:
A) A precipitate of Ca(NO3)2 forms
B) A precipitate of AgI forms
C) Both Ca(NO3)2 and AgI precipitate
D) No precipitate will form
A) A precipitate of Ca(NO3)2 forms
B) A precipitate of AgI forms
C) Both Ca(NO3)2 and AgI precipitate
D) No precipitate will form

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
38
Which of the following equations best represents the balanced net ionic equation for the reaction between aqueous solutions of ammonium phosphate and iron(II)nitrate are mixed?
A) 3 Fe2+(aq)+ 2 PO43-(aq)→ Fe3(PO4)2(s)
B) 2 NH4+(aq)+ Fe(NO3)2(aq)→ 2 NH4NO3(aq)+ Fe2+(aq)
C) 3 Fe2+(aq)+ 2 PO43-(aq)→ Fe3(PO4)2(aq)
D) 2 (NH4)3PO4(aq)+ 3 Fe2+(aq)→ Fe3(PO4)2(s)+ 6 NH4+(aq)
E) 2 (NH4)3PO4(aq)+ 3 Fe(NO3)2(aq)→ Fe3(PO4)2(s)+ 6 NH4NO3(aq)
A) 3 Fe2+(aq)+ 2 PO43-(aq)→ Fe3(PO4)2(s)
B) 2 NH4+(aq)+ Fe(NO3)2(aq)→ 2 NH4NO3(aq)+ Fe2+(aq)
C) 3 Fe2+(aq)+ 2 PO43-(aq)→ Fe3(PO4)2(aq)
D) 2 (NH4)3PO4(aq)+ 3 Fe2+(aq)→ Fe3(PO4)2(s)+ 6 NH4+(aq)
E) 2 (NH4)3PO4(aq)+ 3 Fe(NO3)2(aq)→ Fe3(PO4)2(s)+ 6 NH4NO3(aq)

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
39
What is the balanced equation for carbonate ion (CO32-)acting as a Brønsted base in a reaction with water?
A) CO32-(aq)+ 3 H2O(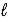 )
)  CO44-(aq)+ 2 H3O+(aq)
CO44-(aq)+ 2 H3O+(aq)
B) CO32-(aq)+ H2O(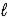 )
) 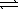 HCO3-(aq)+ OH-(aq)
HCO3-(aq)+ OH-(aq)
C) CO32-(aq)+ H2O(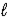 )
) 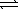 CO2(g)+ 2 OH-(aq)
CO2(g)+ 2 OH-(aq)
D) CO32-(aq)+ 2 H2O(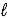 )
) 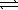 HCO3-(aq)+ H3O+(aq)
HCO3-(aq)+ H3O+(aq)
E) CO32-(aq)+ H2O(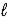 )
) 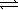 H2CO42-(aq)
H2CO42-(aq)
A) CO32-(aq)+ 3 H2O(
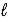 )
) 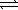 CO44-(aq)+ 2 H3O+(aq)
CO44-(aq)+ 2 H3O+(aq)B) CO32-(aq)+ H2O(
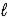 )
) 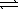 HCO3-(aq)+ OH-(aq)
HCO3-(aq)+ OH-(aq)C) CO32-(aq)+ H2O(
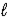 )
) 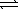 CO2(g)+ 2 OH-(aq)
CO2(g)+ 2 OH-(aq)D) CO32-(aq)+ 2 H2O(
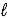 )
) 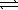 HCO3-(aq)+ H3O+(aq)
HCO3-(aq)+ H3O+(aq)E) CO32-(aq)+ H2O(
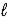 )
) 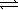 H2CO42-(aq)
H2CO42-(aq)
Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
40
What is the net ionic equation for the reaction of aqueous sodium hydroxide and aqueous iron(II)chloride?
A) Na+(aq)+ OH-(aq)→ NaOH(s)
B) Na+(aq)+ Cl-(aq)→ NaCl(s)
C) Fe2+(aq)+ 2 OH-(aq)→ Fe(OH)2(s)
D) Fe2+(aq)+ OH-(aq)→ FeOH+(s)
E) Fe2+(aq)+ 2 Cl-(aq)→ FeCl2(s)
A) Na+(aq)+ OH-(aq)→ NaOH(s)
B) Na+(aq)+ Cl-(aq)→ NaCl(s)
C) Fe2+(aq)+ 2 OH-(aq)→ Fe(OH)2(s)
D) Fe2+(aq)+ OH-(aq)→ FeOH+(s)
E) Fe2+(aq)+ 2 Cl-(aq)→ FeCl2(s)

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
41
Hydrocyanic acid,HCN,is a weak acid.Write a net ionic equation for the reaction of aqueous hydrocyanic acid and aqueous sodium hydroxide.
A) HCN(aq)+ NaOH(aq)→ Na+(aq)+ CN-(aq)+ H2O(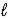 )
)
B) HCN(aq)+ H2O(aq)→ CN-(aq)+ H3O+(aq)
C) H+(aq)+ OH-(aq)→ H2O(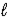 )
)
D) HCN(aq)+ OH-(aq)→ CN-(aq)+ H2O(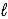 )
)
E) H+(aq)+ NaOH(aq)→ Na+(aq)+ H2O(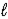 )
)
A) HCN(aq)+ NaOH(aq)→ Na+(aq)+ CN-(aq)+ H2O(
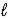 )
)B) HCN(aq)+ H2O(aq)→ CN-(aq)+ H3O+(aq)
C) H+(aq)+ OH-(aq)→ H2O(
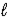 )
)D) HCN(aq)+ OH-(aq)→ CN-(aq)+ H2O(
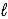 )
)E) H+(aq)+ NaOH(aq)→ Na+(aq)+ H2O(
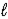 )
)
Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
42
What is the oxidation number of each atom in sodium hydrogen carbonate,NaHCO3?
A) Na = +1,H = -1,C = +6,and O = -2
B) Na = +1,H = +1,C = +4,and O = -2
C) Na = +1,H = -1,C = +2,and O = -2
D) Na = -1,H = +1,C = 0,and O = -2
E) Na = +1,H = -1,C = 0,and O = 0
A) Na = +1,H = -1,C = +6,and O = -2
B) Na = +1,H = +1,C = +4,and O = -2
C) Na = +1,H = -1,C = +2,and O = -2
D) Na = -1,H = +1,C = 0,and O = -2
E) Na = +1,H = -1,C = 0,and O = 0

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
43
What is the oxidation number of P in NH4(H2PO4)?
A) -2
B) -3
C) 5
D) 1
E) 3
A) -2
B) -3
C) 5
D) 1
E) 3

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
44
Which species in the reaction below undergoes reduction? H2O(g)+ CO(g)→ H2(g)+ CO2(g)
A) H2O
B) CO
C) H2
D) CO2
E) None
A) H2O
B) CO
C) H2
D) CO2
E) None

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
45
Which of the following is the correct net ionic equation for the reaction between aqueous ammonia and hydrobromic acid?
A) HBr(aq)+ NH3(aq)→ NH4Br(aq)
B) H+(aq)+ OH-(aq)→ H2O(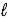 )
)
C) HBr(aq)+ OH-(aq)→ Br-(aq)+ H2O(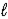 )
)
D) H+(aq)+ NH3(aq)→ NH4+(aq)
E) H+(aq)+ Br-(aq)+ NH3(aq)→ NH4+(aq)+ Br-(aq)
A) HBr(aq)+ NH3(aq)→ NH4Br(aq)
B) H+(aq)+ OH-(aq)→ H2O(
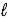 )
)C) HBr(aq)+ OH-(aq)→ Br-(aq)+ H2O(
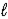 )
)D) H+(aq)+ NH3(aq)→ NH4+(aq)
E) H+(aq)+ Br-(aq)+ NH3(aq)→ NH4+(aq)+ Br-(aq)

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
46
Which species is reduced in the reaction below? I-(aq)+ ClO-(aq)→ IO-(aq)+ Cl-(aq)
A) I-
B) H2O
C) Cl-
D) IO-
E) ClO-
A) I-
B) H2O
C) Cl-
D) IO-
E) ClO-

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
47
Write a balanced chemical equation for the reaction of aqueous solutions of ammonium sulfate and sodium hydroxide.
A) (NH4)2SO4(aq)+ 2 NaOH(aq)→ 2 NH4OH(aq)+ SO3(g)+ Na2O(aq)
B) (NH3)2SO4(aq)+ NaOH(aq)→ 2 NH3(g)+ NaOHSO4(aq)
C) (NH3)2SO4(aq)+ 2 NaOH(aq)→ 2 NH3(g)+ Na2SO4(aq)+ 2 OH−(aq)
D) (NH4)2SO4(aq)+ 2 NaOH(aq)→ 2 NH4+(g)+ Na2SO4(aq)+ 2 OH−(aq)
E) (NH4)2SO4(aq)+ 2 NaOH(aq)→ 2 NH3(g)+ 2 H2O(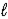 )+ Na2SO4(aq)
)+ Na2SO4(aq)
A) (NH4)2SO4(aq)+ 2 NaOH(aq)→ 2 NH4OH(aq)+ SO3(g)+ Na2O(aq)
B) (NH3)2SO4(aq)+ NaOH(aq)→ 2 NH3(g)+ NaOHSO4(aq)
C) (NH3)2SO4(aq)+ 2 NaOH(aq)→ 2 NH3(g)+ Na2SO4(aq)+ 2 OH−(aq)
D) (NH4)2SO4(aq)+ 2 NaOH(aq)→ 2 NH4+(g)+ Na2SO4(aq)+ 2 OH−(aq)
E) (NH4)2SO4(aq)+ 2 NaOH(aq)→ 2 NH3(g)+ 2 H2O(
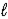 )+ Na2SO4(aq)
)+ Na2SO4(aq)
Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
48
Write a balanced net ionic equation for the reaction of aqueous solutions of baking soda (NaHCO3)and acetic acid.
A) HCO3-(aq)+ CH3CO2H(aq)→ CH3CO2-(aq)+ H2O(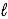 )+ CO2(g)
)+ CO2(g)
B) 2 NaHCO3(aq)+ CH3CO2H(aq)→ 2 Na2CO3(aq)+ CH4(aq)+ 2H2O(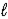 )+ CO2(g)
)+ CO2(g)
C) NaHCO3(aq)+ H+(aq)→ H2CO3(s)+ Na+(aq)
D) HCO3-(aq)+ H+(aq)→ H2O(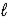 )+ CO2(g)
)+ CO2(g)
E) HCO3-(aq)+ H+(aq)→ H2CO3(aq)
A) HCO3-(aq)+ CH3CO2H(aq)→ CH3CO2-(aq)+ H2O(
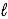 )+ CO2(g)
)+ CO2(g)B) 2 NaHCO3(aq)+ CH3CO2H(aq)→ 2 Na2CO3(aq)+ CH4(aq)+ 2H2O(
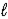 )+ CO2(g)
)+ CO2(g)C) NaHCO3(aq)+ H+(aq)→ H2CO3(s)+ Na+(aq)
D) HCO3-(aq)+ H+(aq)→ H2O(
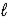 )+ CO2(g)
)+ CO2(g)E) HCO3-(aq)+ H+(aq)→ H2CO3(aq)

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
49
Which molecule in the reaction below is the oxidizing agent? 2 C2H6(g)+ 7 O2(g)→ 4 CO2(g)+ 6 H2O(g)
A) C2H6
B) O2
C) H2O
D) CO2
E) None
A) C2H6
B) O2
C) H2O
D) CO2
E) None

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
50
Which of the following compounds will produce a basic solution when dissolved in water?
A) CaO
B) NaHSO4
C) CO2
D) SO2
E) KCl
A) CaO
B) NaHSO4
C) CO2
D) SO2
E) KCl

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
51
What are the spectator ions in the reaction between aqueous hydroiodic acid and aqueous sodium hydroxide?
A) Na+ only
B) H+ and OH-
C) Na+ and I-
D) I- only
E) H+,I-,Na+,and OH-
A) Na+ only
B) H+ and OH-
C) Na+ and I-
D) I- only
E) H+,I-,Na+,and OH-

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
52
The net ionic equation for the reaction of calcium carbonate with nitric acid is
A) CaCO3(s)+ 2H+(aq)→ Ca2+(aq)+ CO2(g)+ H2O(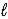 ).
).
B) Ca2+(aq)+ CO32-(aq)+ 2H+(aq)+ 2NO3-(aq)→ Ca(NO3)2(aq)+ CO2(g)+ H2O(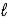 ).
).
C) CaCO3(s)+ 2HNO2(aq)→ Ca2+(aq)+ 2NO2-(aq)+ CO2(g)+ H2O(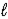 ).
).
D) Ca(HCO3)2(s)+ 2HNO3(aq)→ Ca2+(aq)+ 2NO3-(aq)+ CO2(g)+ 2H2O(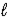 ).
).
E) CaCO3(s)+ 2HNO3(aq)→ Ca2+(aq)+ 2NO3-(aq)+ CO2(g)+ H2O(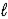 ).
).
A) CaCO3(s)+ 2H+(aq)→ Ca2+(aq)+ CO2(g)+ H2O(
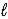 ).
).B) Ca2+(aq)+ CO32-(aq)+ 2H+(aq)+ 2NO3-(aq)→ Ca(NO3)2(aq)+ CO2(g)+ H2O(
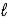 ).
).C) CaCO3(s)+ 2HNO2(aq)→ Ca2+(aq)+ 2NO2-(aq)+ CO2(g)+ H2O(
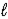 ).
).D) Ca(HCO3)2(s)+ 2HNO3(aq)→ Ca2+(aq)+ 2NO3-(aq)+ CO2(g)+ 2H2O(
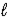 ).
).E) CaCO3(s)+ 2HNO3(aq)→ Ca2+(aq)+ 2NO3-(aq)+ CO2(g)+ H2O(
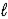 ).
).
Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
53
What is the net ionic equation for the neutralization of hydrochloric acid with aqueous potassium hydroxide?
A) H+(aq)+ OH-(aq)→ H2O(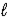 )
)
B) H+(aq)+ Cl-(aq)+ K+(aq)+ OH-(aq)→ KCl(aq)+ H2O(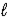 )
)
C) H+(aq)+ Cl-(aq)+ K+(aq)+ OH-(aq)→ HCl(aq)+ KOH(aq)
D) HCl(aq)+ K+(aq)+ OH-(aq)→ KCl(aq)+ H2O(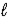 )
)
E) HCl(aq)+ KOH(aq)→ KCl(aq)+ H2O(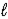 )
)
A) H+(aq)+ OH-(aq)→ H2O(
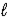 )
)B) H+(aq)+ Cl-(aq)+ K+(aq)+ OH-(aq)→ KCl(aq)+ H2O(
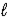 )
)C) H+(aq)+ Cl-(aq)+ K+(aq)+ OH-(aq)→ HCl(aq)+ KOH(aq)
D) HCl(aq)+ K+(aq)+ OH-(aq)→ KCl(aq)+ H2O(
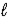 )
)E) HCl(aq)+ KOH(aq)→ KCl(aq)+ H2O(
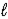 )
)
Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
54
What is the oxidation number of Fe in BaFeO4?
A) 2
B) -2
C) 6
D) +3
E) 0
A) 2
B) -2
C) 6
D) +3
E) 0

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
55
In the reaction below,which species is oxidized? 3 Rb2S(s)+ 8 H+(aq)+ 2 NO3-(aq)→ 6 Rb+(aq)+ 3 S(s)+ 2 NO(g)+ 4 H2O
A) NO3-
B) Rb2S
C) Rb+
D) NO
E) H+
A) NO3-
B) Rb2S
C) Rb+
D) NO
E) H+

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
56
Metal oxides react with water to produce ____.
A) bases
B) hydrogen gas
C) oxygen gas
D) acids
E) hydronium ions
A) bases
B) hydrogen gas
C) oxygen gas
D) acids
E) hydronium ions

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
57
In the reaction of acetic acid with aqueous lithium hydroxide,what is the spectator ion?
A) OH-(aq)
B) There is no spectator ion.
C) C2H3O2-(aq)
D) Li+(aq)
E) H+(aq)
A) OH-(aq)
B) There is no spectator ion.
C) C2H3O2-(aq)
D) Li+(aq)
E) H+(aq)

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
58
Which of the following is a weak electrolyte in aqueous solution?
A) Mg(OH)2
B) NH3
C) LiOH
D) KOH
E) Sr(OH)2
A) Mg(OH)2
B) NH3
C) LiOH
D) KOH
E) Sr(OH)2

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
59
Which of the following is/are spectator ions in the reaction between aqueous nitric acid and ammonia?
A) H+
B) NO3-
C) H+ and NH4+
D) NO3- and NH4+
E) H+,NO3-,and NH4+
A) H+
B) NO3-
C) H+ and NH4+
D) NO3- and NH4+
E) H+,NO3-,and NH4+

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
60
The oxidation number of chlorine is highest in which of the following?
A) HCl
B) Cl2
C) HClO2
D) NaClO3
E) ClO2−
A) HCl
B) Cl2
C) HClO2
D) NaClO3
E) ClO2−

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
61
Which of the following is not an oxidation−reduction reaction?
A) CaCO3(s)→ CaO(s)+ CO2(g)
B) 2 Na(s)+ Br2(g)→ 2 NaBr(g)
C) Fe(s)+ 2 HCl(aq)→ FeCl2(aq)+ H2(g)
D) 2 C(s)+ O2(g)→ 2 CO(g)
E) 2 H2O(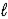 )→ 2 H2(g)+ O2(g)
)→ 2 H2(g)+ O2(g)
A) CaCO3(s)→ CaO(s)+ CO2(g)
B) 2 Na(s)+ Br2(g)→ 2 NaBr(g)
C) Fe(s)+ 2 HCl(aq)→ FeCl2(aq)+ H2(g)
D) 2 C(s)+ O2(g)→ 2 CO(g)
E) 2 H2O(
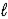 )→ 2 H2(g)+ O2(g)
)→ 2 H2(g)+ O2(g)
Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
62
Identify a true statement about oxidation numbers.
A) Each atom in an element has an oxidation number of 1.
B) For monoatomic ions,the oxidation number is equal to the charge on the ion.
C) The oxidation number of H is 0 in most of the compounds.
D) The oxidation number of O is +2 in most of the compounds.
E) Fluorine always has an oxidation number of +1 in most of the compounds.
A) Each atom in an element has an oxidation number of 1.
B) For monoatomic ions,the oxidation number is equal to the charge on the ion.
C) The oxidation number of H is 0 in most of the compounds.
D) The oxidation number of O is +2 in most of the compounds.
E) Fluorine always has an oxidation number of +1 in most of the compounds.

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
63
Which of the following chemical equations is an acid-base reaction?
A) Ba(OH)2(aq)+ K2SO4(aq)→ BaSO4(s)+ 2 KOH(aq)
B) 3 NaOH(aq)+ AlCl3(aq)→ Al(OH)3(s)+ 3 NaCl(aq)
C) 2 H+(aq)+ Zn(s)→ H2(g)+ Zn2+(aq)
D) 2 HCl(aq)+ Pb(NO3)2(aq)→ PbCl2(s)+ 2 HNO3(aq)
E) H3PO4(aq)+ NH3(aq)→ NH4+(aq)+ H2PO4−(aq)
A) Ba(OH)2(aq)+ K2SO4(aq)→ BaSO4(s)+ 2 KOH(aq)
B) 3 NaOH(aq)+ AlCl3(aq)→ Al(OH)3(s)+ 3 NaCl(aq)
C) 2 H+(aq)+ Zn(s)→ H2(g)+ Zn2+(aq)
D) 2 HCl(aq)+ Pb(NO3)2(aq)→ PbCl2(s)+ 2 HNO3(aq)
E) H3PO4(aq)+ NH3(aq)→ NH4+(aq)+ H2PO4−(aq)

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
64
Which of the following elements generally acts as an oxidizing agent?
A) Br2
B) H2
C) Fe
D) C
E) Li
A) Br2
B) H2
C) Fe
D) C
E) Li

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
65
Ionic and molecular compounds that form ions in aqueous solution are called _____.

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
66
A(n)_____ agent gains electrons in an oxidation-reduction reaction.

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
67
If an aqueous sodium hydroxide solution is left in contact with air,the concentration of hydroxide ion gradually decreases.The process can be hastened if a person exhales over a sodium hydroxide solution.Write a balanced chemical equation that describes the process by which the hydroxide ion concentration decreases.

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
68
Give the name of an acidic oxide and write a balanced chemical equation for the reaction of the oxide with water.

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
69
A solution is a homogeneous mixture composed of one or more ____________ dissolved in a solvent.

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
70
The net ionic equation for the reaction of barium chloride and sodium sulfate is shown below.
Ba2+(aq)+ SO42-(aq)→ BaSO4(s)
Chloride and sodium ions are referred to as ________ ions because they are not involved in the reaction.
Ba2+(aq)+ SO42-(aq)→ BaSO4(s)
Chloride and sodium ions are referred to as ________ ions because they are not involved in the reaction.

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
71
________ acid is produced in a larger quantity than any other chemical in the United States.This chemical is used in the production of fertilizers,pigments,alcohol,paper and detergents.

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck
72
_____-oxides produce acids when reacted with water.

Unlock Deck
Unlock for access to all 72 flashcards in this deck.
Unlock Deck
k this deck



