Deck 16: Principles of Chemical Reactivity: Equilibria
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/75
Play
Full screen (f)
Deck 16: Principles of Chemical Reactivity: Equilibria
1
Given the following chemical equilibrium
COCl2(g) CO(g) + Cl2(g), calculate the value of Kc when Kp = 6.5 × 1011 at 298 K. (R = 0.08206 L?atm/mol ? K)
A) 1.5 × 10-12
B) 3.8 × 10-11
C) 1.1 × 109
D) 2.7 × 1010
E) 1.6 × 1013
COCl2(g) CO(g) + Cl2(g), calculate the value of Kc when Kp = 6.5 × 1011 at 298 K. (R = 0.08206 L?atm/mol ? K)
A) 1.5 × 10-12
B) 3.8 × 10-11
C) 1.1 × 109
D) 2.7 × 1010
E) 1.6 × 1013
2.7 × 1010
2
Which of the following is the correct balanced equation for the equilibrium expression given below?
A) 2 SO2(g) + O2(g) 2 SO3(g)
B) 2 SO3(g) 2 SO2(g) + O2(g)
C) 2 SO3(aq) 2 SO2(aq) + O2(aq)
D) 2 SO2(aq) + O2(aq) 2 SO3(aq)
E) SO2(g) + O2(g) SO3(g)
A) 2 SO2(g) + O2(g) 2 SO3(g)
B) 2 SO3(g) 2 SO2(g) + O2(g)
C) 2 SO3(aq) 2 SO2(aq) + O2(aq)
D) 2 SO2(aq) + O2(aq) 2 SO3(aq)
E) SO2(g) + O2(g) SO3(g)
2 SO2(g) + O2(g) 2 SO3(g)
3
Which of the following is always true for a reaction where
Kc is at 25°C?
A) The reaction mixture contains mostly reactants at equilibrium.
B) The reaction mixture contains mostly products at equilibrium.
C) The rate of reaction is very slow.
D) There are approximately equal moles of reactants and products at equilibrium.
E) Both A and C.
Kc is at 25°C?
A) The reaction mixture contains mostly reactants at equilibrium.
B) The reaction mixture contains mostly products at equilibrium.
C) The rate of reaction is very slow.
D) There are approximately equal moles of reactants and products at equilibrium.
E) Both A and C.
The reaction mixture contains mostly reactants at equilibrium.
4
Which of the following expressions for K is correct for the reaction given below?
HF(aq) + H2O( ) F-(aq) + H3O+(aq)
A)
B)
C)
D)
E)
HF(aq) + H2O( ) F-(aq) + H3O+(aq)
A)
B)
C)
D)
E)

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
5
Write the expression for Kp for the reaction below.
2 NOBr(g) 2 NO(g) + Br2( )
A)
B)
C)
D)
E)
2 NOBr(g) 2 NO(g) + Br2( )
A)
B)
C)
D)
E)

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
6
For which of the following reactions is Kc = Kp?
A) N2(g) + 3 H2(g) 2 NH3(g)
B) CO(g) + H2O(g) CO2(g) + H2(g)
C) CO(g) + 3 H2(g) CH4(g) + H2O(g)
D) CaO(s) + CO2(g) CaCO3(s)
E) HBr(g) ½ H2(g) + ½ Br2( )
A) N2(g) + 3 H2(g) 2 NH3(g)
B) CO(g) + H2O(g) CO2(g) + H2(g)
C) CO(g) + 3 H2(g) CH4(g) + H2O(g)
D) CaO(s) + CO2(g) CaCO3(s)
E) HBr(g) ½ H2(g) + ½ Br2( )

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
7
The reaction quotient, Q, for a system is . If the equilibrium constant for the system at some temperature is , what will happen as the reaction mixture returns to equilibrium?
A) The equilibrium constant will increase until it equals the reaction quotient.
B) There will be a net loss in both product(s) and reactant(s).
C) There will be a net loss in product(s).
D) There will be a net loss in reactant(s).
E) The equilibrium constant will increase.
A) The equilibrium constant will increase until it equals the reaction quotient.
B) There will be a net loss in both product(s) and reactant(s).
C) There will be a net loss in product(s).
D) There will be a net loss in reactant(s).
E) The equilibrium constant will increase.

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
8
Which of the following is the correct balanced equation for the equilibrium constant expression given below?
A) PbF2(aq) Pb(s) + F2(aq)
B) PbF2(s) Pb2+(aq) + 2 F-(aq)
C) Pb2+(aq) + 2 F-(aq) PbF2(s)
D) Pb(s) + F2(aq) PbF2(aq)
E) PbF+(aq) + F-(aq) PbF2(aq)
A) PbF2(aq) Pb(s) + F2(aq)
B) PbF2(s) Pb2+(aq) + 2 F-(aq)
C) Pb2+(aq) + 2 F-(aq) PbF2(s)
D) Pb(s) + F2(aq) PbF2(aq)
E) PbF+(aq) + F-(aq) PbF2(aq)

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
9
Write a balanced chemical equation which corresponds to the following equilibrium constant expression.
A) HNO2(aq) + H2O( ) NO2-(aq) + H3O+(aq)
B) NO2-(aq) + H3O+(aq) HNO2(aq) + H2O( )
C) NO2-(aq) + H3O+(aq) HNO2(aq)
D) H+(aq) + OH-(aq) H2O( )
E) HNO2(aq) NO2-(aq) + H3O+(aq)
A) HNO2(aq) + H2O( ) NO2-(aq) + H3O+(aq)
B) NO2-(aq) + H3O+(aq) HNO2(aq) + H2O( )
C) NO2-(aq) + H3O+(aq) HNO2(aq)
D) H+(aq) + OH-(aq) H2O( )
E) HNO2(aq) NO2-(aq) + H3O+(aq)

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
10
For which of the following reactions is Kp = Kc?
A) 2 CO2(g) 2 CO(g) + O2(g)
B) CH4(g) + 2 O2(g) CO2(g) + 2 H2O(g)
C) C(s) + H2O(g) H2(g) + CO(g)
D) NH3(g) 3/2 H2(g) + 1/2 N2(g)
E) 2 O3(g) 3 O2(g)
A) 2 CO2(g) 2 CO(g) + O2(g)
B) CH4(g) + 2 O2(g) CO2(g) + 2 H2O(g)
C) C(s) + H2O(g) H2(g) + CO(g)
D) NH3(g) 3/2 H2(g) + 1/2 N2(g)
E) 2 O3(g) 3 O2(g)

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
11
Which of the following expressions for K is correct for the reaction given below? Al3+(aq) + 4 OH-(aq) Al(OH)4-(aq)
A)
B)
C)
D)
E)
A)
B)
C)
D)
E)

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
12
For the reaction 2NO(g) + O2(g) 2NO2(g) at 750°C, what is the relationship between Kc and Kp?
A) Kc = Kp
B) Kc = Kp × (RT)-1
C) Kc = Kp = 1.0
D) Kc = Kp × (RT)¾
E) Kc = Kp × (RT)1
A) Kc = Kp
B) Kc = Kp × (RT)-1
C) Kc = Kp = 1.0
D) Kc = Kp × (RT)¾
E) Kc = Kp × (RT)1

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
13
If the reaction quotient, Q, is equal to K in a gas phase reaction, then
A) the chemical system has reached equilibrium.
B) the temperature must be increased for the reaction to proceed in the forward direction.
C) the reaction will proceed in the forward direction until equilibrium is established.
D) the reaction will proceed in the backward direction until equilibrium is established.
E) the reaction will proceed in the direction that increases the number of gas phase particles.
A) the chemical system has reached equilibrium.
B) the temperature must be increased for the reaction to proceed in the forward direction.
C) the reaction will proceed in the forward direction until equilibrium is established.
D) the reaction will proceed in the backward direction until equilibrium is established.
E) the reaction will proceed in the direction that increases the number of gas phase particles.

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
14
If the reaction quotient, Q, is greater than K in a gas phase reaction, then
A) the chemical system has reached equilibrium.
B) the temperature must be increased for the reaction to proceed in the forward direction.
C) the reaction will proceed in the forward direction until equilibrium is established.
D) the reaction will proceed in the backward direction until equilibrium is established.
E) the reaction will proceed in the direction that increases the number of gas phase particles.
A) the chemical system has reached equilibrium.
B) the temperature must be increased for the reaction to proceed in the forward direction.
C) the reaction will proceed in the forward direction until equilibrium is established.
D) the reaction will proceed in the backward direction until equilibrium is established.
E) the reaction will proceed in the direction that increases the number of gas phase particles.

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
15
Consider the formation of ozone by the following reaction.
3 O2(g) 2 O3(g)
Calculate the value of
Kp, given that Kc = 2.5 × 10-29 at 298 K. (R = 0.08206 L?atm/mol ? K)
A) 1.0 × 10-30
B) 2.1 × 10-30
C) 2.5 × 10-29
D) 3.3 × 10-28
E) 6.1 ×10-28
3 O2(g) 2 O3(g)
Calculate the value of
Kp, given that Kc = 2.5 × 10-29 at 298 K. (R = 0.08206 L?atm/mol ? K)
A) 1.0 × 10-30
B) 2.1 × 10-30
C) 2.5 × 10-29
D) 3.3 × 10-28
E) 6.1 ×10-28

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
16
What is the Kc equilibrium-constant expression for the following equilibrium? NiO(s) + H2(g) Ni(s) + H2O(g)
A)
B) ?
C) ?
D) ?
E) ?
A)
B) ?
C) ?
D) ?
E) ?

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
17
What is the Kc expression for the equilibrium given below?
CuI(s) Cu+(aq) + I?(aq)
A)
B)
C)
D)
E)
CuI(s) Cu+(aq) + I?(aq)
A)
B)
C)
D)
E)

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
18
What is the expression for Kc for the following equilibrium?
CaSO3(s) CaO(s) + SO2(g)
A)
B) ?
C) ?
D) ?
E) ?
CaSO3(s) CaO(s) + SO2(g)
A)
B) ?
C) ?
D) ?
E) ?

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
19
Which of the following is the correct balanced equation for the equilibrium constant expression given below?
A) H2(g) + I2(g) HI(g)
B) HI(g) H2(g) + I2(g)
C) H2(aq) + I2(aq) HI(aq)
D) HI(aq) H2(aq) + I2(aq)
E) 2 HI(g) H2(g) + I2(g)
A) H2(g) + I2(g) HI(g)
B) HI(g) H2(g) + I2(g)
C) H2(aq) + I2(aq) HI(aq)
D) HI(aq) H2(aq) + I2(aq)
E) 2 HI(g) H2(g) + I2(g)

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
20
Which of the following expressions correctly describes the equilibrium constant Kc for the reaction given below?
2 C2H2(g) + 5 O2(g) 4 CO2(g) + 2 H2O(g)
A)
B)
C)
D)
E)
2 C2H2(g) + 5 O2(g) 4 CO2(g) + 2 H2O(g)
A)
B)
C)
D)
E)

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
21
An aqueous mixture of phenol and ammonia has initial concentrations of 0.200 M C6H5OH(aq) and 0.120 M NH3(aq). At equilibrium, the C6H5O-(aq) concentration is 0.050 M. Calculate the equilibrium constant, K, for the reaction below. C6H5OH(aq) + NH3(aq) C6H5O- + NH4+(aq)
A) 0.10
B) 0.24
C) 2.1
D) 4.2
E) 4.8
A) 0.10
B) 0.24
C) 2.1
D) 4.2
E) 4.8

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
22
A 3.50-mol sample of HI is placed in a 1.00-L vessel at 460°C, and the reaction system is allowed to come to equilibrium. The HI partially decomposes, forming 0.266 mol H2 and 0.266 mol I2 at equilibrium. What is the equilibrium constant Kc for the following reaction at 460°C?
½ H2(g) + ½ I2(g) HI(g)
A) 1.23 × 102
B) 8.10 × 10-3
C) 2.40 × 10-2
D) 11.1
E) 6.45
½ H2(g) + ½ I2(g) HI(g)
A) 1.23 × 102
B) 8.10 × 10-3
C) 2.40 × 10-2
D) 11.1
E) 6.45

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
23
For the reaction given below, 2.00 moles of A and 3.00 moles of B are placed in a 6.00-L container. A(g) + 2B(g) C(g)
At equilibrium, the concentration of A is 0.230 mol/L. What is the value of Kc?
A) 1.20
B) 1.53
C) 5.22
D) 0.230
E) 0.449
At equilibrium, the concentration of A is 0.230 mol/L. What is the value of Kc?
A) 1.20
B) 1.53
C) 5.22
D) 0.230
E) 0.449

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
24
Consider the reaction below. 2 HF(g) H2(g) + F2(g) (Kc = 1.00 × 10-2)
Given that 1.00 mol of HF(g), 0.241 mol of H2(g), and 0.750 mol of F2(g) are mixed in a 5.00 L flask, determine the reaction quotient, Q.
A) Q = 0.0452
B) Q = 0.181
C) Q = 0.0362
D) Q = 1.99
E) None of these
Given that 1.00 mol of HF(g), 0.241 mol of H2(g), and 0.750 mol of F2(g) are mixed in a 5.00 L flask, determine the reaction quotient, Q.
A) Q = 0.0452
B) Q = 0.181
C) Q = 0.0362
D) Q = 1.99
E) None of these

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
25
Consider the following equilibrium:
C2H6(g) + C5H12(g) CH4(g) + C6H14(g); Kp = 9.57 at 500 K
Suppose 22.7 g each of CH4, C2H6, C5H12, and C6H14 are placed in a 35.0-L reaction vessel at 500 K. Which of the following statements is correct?
A) Because Qc < Kc, more products will be formed.
B) Because Qc = 1, the system is at equilibrium.
C) Because Qc = 1, more products will be formed.
D) Because Qc = 1, more reactants will be formed.
E) Because Qc > Kc, more reactants will be formed.
C2H6(g) + C5H12(g) CH4(g) + C6H14(g); Kp = 9.57 at 500 K
Suppose 22.7 g each of CH4, C2H6, C5H12, and C6H14 are placed in a 35.0-L reaction vessel at 500 K. Which of the following statements is correct?
A) Because Qc < Kc, more products will be formed.
B) Because Qc = 1, the system is at equilibrium.
C) Because Qc = 1, more products will be formed.
D) Because Qc = 1, more reactants will be formed.
E) Because Qc > Kc, more reactants will be formed.

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
26
Which of the following statements about the reaction quotient, Q, is false?
A) The value of Q can be used to predict equilibrium concentrations.
B) It has the same expression as Kc.
C) Its value is calculated using nonequilibrium concentrations.
D) If Q > Kc, the reaction must move to equilibrium by forming more reactants.
E) If Q < Kc, the reaction must move to equilibrium by forming more products.
A) The value of Q can be used to predict equilibrium concentrations.
B) It has the same expression as Kc.
C) Its value is calculated using nonequilibrium concentrations.
D) If Q > Kc, the reaction must move to equilibrium by forming more reactants.
E) If Q < Kc, the reaction must move to equilibrium by forming more products.

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
27
The following reaction occurred when a 1.0-liter reaction vessel was initially charged with 2.0 moles of N2(g) and 4.0 moles of H2(g): 3H2(g) + N2(g) 2NH3(g)
Once equilibrium was established, the concentration of NH3(g) was determined to be 0.57 M at 700.°C. The value for Kc at 700.°C for the formation of ammonia is:
A) 3.2 × 10-1
B) 6.1 × 10-3
C) 1.1 × 10-1
D) 6.0 × 10-2
E) none of these
Once equilibrium was established, the concentration of NH3(g) was determined to be 0.57 M at 700.°C. The value for Kc at 700.°C for the formation of ammonia is:
A) 3.2 × 10-1
B) 6.1 × 10-3
C) 1.1 × 10-1
D) 6.0 × 10-2
E) none of these

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
28
Exactly 1.0 mol N2O4 is placed in an empty 1.0-L container and allowed to reach equilibrium described by the equation N2O4(g) 2NO2(g). If at equilibrium the N2O4 is 27.0% dissociated, what is the value of the equilibrium constant, Kc, for the reaction under these conditions?
A) 0.40
B) 2.5
C) 0.29
D) 0.74
E) 0.10
A) 0.40
B) 2.5
C) 0.29
D) 0.74
E) 0.10

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
29
A 10.0 g sample of solid NH4Cl is heated in a 5.00 L container to 900 °C. At equilibrium, the pressure of NH3(g) is 1.17 atm. Calculate the equilibrium constant, Kp, for the reaction below. NH4Cl(s) NH3(g) + HCl(g)
A) 1.37
B) 4.93
C) 1.17
D) 2.34
E) None of these
A) 1.37
B) 4.93
C) 1.17
D) 2.34
E) None of these

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
30
A 2.5 L flask is filled with 0.25 mol SO3, 0.20 mol SO2, and 0.40 mol O2, and allowed to reach equilibrium. Assume the temperature of the mixture is chosen so that Kc = 0.12. Predict the effect on the concentration of SO3 as equilibrium is achieved by using Q, the reaction quotient. 2 SO3(g) 2 SO2(g) + O2(g)
A) [SO3] will decrease because Q > K.
B) [SO3] will decrease because Q < K.
C) [SO3] will increase because Q < K.
D) [SO3] will increase because Q > K.
E) [SO3] will remain the same because Q = K.
A) [SO3] will decrease because Q > K.
B) [SO3] will decrease because Q < K.
C) [SO3] will increase because Q < K.
D) [SO3] will increase because Q > K.
E) [SO3] will remain the same because Q = K.

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
31
Consider the reaction A(aq) 2 B(aq) where Kc = 4.1 at 25 °C. If 0.50 M A(aq) and 1.5 M B(aq) are initially present in a 1.0 L flask at 25 °C, what change in concentrations (if any) will occur in time?
A) [A] will decrease and [B] will decrease.
B) [A] will decrease and [B] will increase.
C) [A] will increase and [B] will decrease.
D) [A] will increase and [B] will increase.
E) [A] and [B] remain unchanged.
A) [A] will decrease and [B] will decrease.
B) [A] will decrease and [B] will increase.
C) [A] will increase and [B] will decrease.
D) [A] will increase and [B] will increase.
E) [A] and [B] remain unchanged.

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
32
What is the reaction quotient, Q, for the equilibrium
CuCl(s) Cu+(aq) + Cl?(aq)
When 0.3746 L of M Cu+ is combined with 0.4326 L of M Cl? in the presence of an excess of CuCl(s)?
A)
B)
C)
D)
E)
CuCl(s) Cu+(aq) + Cl?(aq)
When 0.3746 L of M Cu+ is combined with 0.4326 L of M Cl? in the presence of an excess of CuCl(s)?
A)
B)
C)
D)
E)

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
33
At a high temperature, equal concentrations of 0.160 mol/L of H2(g) and I2(g) are initially present in a flask. The H2 and I2 react according to the balanced equation below. H2(g) + I2(g) 2 HI(g)
When equilibrium is reached, the concentration of H2(g) has decreased to 0.036 mol/L. What is the equilibrium constant, Kc, for the reaction?
A) 3.4
B) 4.0
C) 12
D) 22
E) 48
When equilibrium is reached, the concentration of H2(g) has decreased to 0.036 mol/L. What is the equilibrium constant, Kc, for the reaction?
A) 3.4
B) 4.0
C) 12
D) 22
E) 48

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
34
At a given temperature, 0.0664 mol N2O4(g) is placed in a 1.00 L flask. After reaching equilibrium, the concentration of NO2(g) is 6.1 × 10-3 M. What is Kc for the reaction below?
N2O4(g) 2 NO2(g)
A) 3.7 ×10-5
B) 1.4 ×10-4
C) 5.9 × 10-4
D) 9.6 × 10-2
E) 1.8 × 103
N2O4(g) 2 NO2(g)
A) 3.7 ×10-5
B) 1.4 ×10-4
C) 5.9 × 10-4
D) 9.6 × 10-2
E) 1.8 × 103

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
35
A 3.00-liter flask initially contains 3.00 mol of gas A and 1.50 mol of gas B. Gas A decomposes according to the following reaction: 3A 2B + C
The equilibrium concentration of gas C is 0.134 mol/L. Determine the value of the equilibrium constant, Kc.
A) 0.172
B) 0.132
C) 3.19 × 10-3
D) 0.370
E) none of these
The equilibrium concentration of gas C is 0.134 mol/L. Determine the value of the equilibrium constant, Kc.
A) 0.172
B) 0.132
C) 3.19 × 10-3
D) 0.370
E) none of these

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
36
Nitrogen trifluoride decomposes at to form nitrogen and fluorine gases according to the following equation: 2NF3(g) N2(g) + 3F2(g)
2)50-L reaction vessel is initially charged with 1.22 mol of NF3 and allowed to come to equilibrium at 800 K. Once equilibrium is established, the reaction vessel is found to contain 0.0194 mol of N2. What is the value of Kp at this temperature? (R = 0.0821 L?atm/mol?K)
A) 2.68 × 10-6
B) 1.91 × 10-3
C) 1.79 × 10-3
D) 2.77 × 10-6
E) 4.43 × 10-7
2)50-L reaction vessel is initially charged with 1.22 mol of NF3 and allowed to come to equilibrium at 800 K. Once equilibrium is established, the reaction vessel is found to contain 0.0194 mol of N2. What is the value of Kp at this temperature? (R = 0.0821 L?atm/mol?K)
A) 2.68 × 10-6
B) 1.91 × 10-3
C) 1.79 × 10-3
D) 2.77 × 10-6
E) 4.43 × 10-7

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
37
At 25 °C, 0.138 mg AgBr dissolves in 10.0 L of water. What is the equilibrium constant for the reaction below?
AgBr(s) Ag+(aq) + Br-(aq)
A) 5.40 × 10-13
B) 5.40 × 10-11
C) 1.90 × 10-8
D) 7.35 × 10-7
E) 1.90 × 10-6
AgBr(s) Ag+(aq) + Br-(aq)
A) 5.40 × 10-13
B) 5.40 × 10-11
C) 1.90 × 10-8
D) 7.35 × 10-7
E) 1.90 × 10-6

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
38
Excess Ag2SO4(s) is placed in water at 25 °C. At equilibrium, the solution contains 0.029 M Ag+(aq). What is the equilibrium constant for the reaction below?
Ag2SO4(s) 2 Ag+(aq) + SO42-(aq)
A) 1.8 × 10-7
B) 6.1 × 10-6
C) 1.2 × 10-5
D) 2.4 × 10-5
E) 8.4 × 10-4
Ag2SO4(s) 2 Ag+(aq) + SO42-(aq)
A) 1.8 × 10-7
B) 6.1 × 10-6
C) 1.2 × 10-5
D) 2.4 × 10-5
E) 8.4 × 10-4

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
39
When 0.20 mole HF is dissolved in water to a volume of 1.00 L, 5.8% of the HF dissociates to form F-(aq). What is the equilibrium constant for the reaction? HF(aq) + H2O( ) F-(aq) + H3O+(aq)
A) 1.3 × 10-4
B) 7.1 × 10-4
C) 1.2 × 10-2
D) 1.7 × 10-2
E) 6.2 × 10-2
A) 1.3 × 10-4
B) 7.1 × 10-4
C) 1.2 × 10-2
D) 1.7 × 10-2
E) 6.2 × 10-2

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
40
A sample of solid NH4NO3 was placed in an evacuated container and then heated so that it decomposed explosively according to the following equation: NH4NO3(s) N2O(g) + 2H2O(g)
At equilibrium the total pressure in the container was found to be 2.72 atm at a temperature of 500.°C. Calculate Kp.
A) 1.64
B) 0.822
C) 2.98
D) 80.5
E) 0.745
At equilibrium the total pressure in the container was found to be 2.72 atm at a temperature of 500.°C. Calculate Kp.
A) 1.64
B) 0.822
C) 2.98
D) 80.5
E) 0.745

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
41
Given the following chemical equilibria, N2(g) + O2(g) 2 NO(g)
K1
N2(g) + 3 H2(g) 2 NH3(g)
K2
H2(g) + 1/2 O2(g) H2O(g)
K3
Determine the method used to calculate the equilibrium constant for the reaction below.
4 NH3(g) + 5 O2(g) 4 NO(g) + 6 H2O(g)
K c
A)
B)
C)
D)
E)
K1
N2(g) + 3 H2(g) 2 NH3(g)
K2
H2(g) + 1/2 O2(g) H2O(g)
K3
Determine the method used to calculate the equilibrium constant for the reaction below.
4 NH3(g) + 5 O2(g) 4 NO(g) + 6 H2O(g)
K c
A)
B)
C)
D)
E)

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
42
Given the equilibrium constants for the following reactions:
4Cu(s) + O2(g) 2Cu2O(s), K1
4CuO(s) 2Cu2O(s) + O2(g), K2
What is K for the system
2Cu(s) + O2(g) 2CuO(s)
Equivalent to?
A)
B)
C) (K1)(K2)
D)
E)
4Cu(s) + O2(g) 2Cu2O(s), K1
4CuO(s) 2Cu2O(s) + O2(g), K2
What is K for the system
2Cu(s) + O2(g) 2CuO(s)
Equivalent to?
A)
B)
C) (K1)(K2)
D)
E)

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
43
In an experiment, 0.46 mol H2 and 0.46 mol I2 are mixed in a 1.00-L container, and the reaction forms HI. If Kc = 49. for this reaction, what is the equilibrium concentration of HI? I2(g) + H2(g) 2HI(g)
A) 0.88 M
B) 0.81 M
C) 0.72 M
D) 0.115 M
E) 0.061 M
A) 0.88 M
B) 0.81 M
C) 0.72 M
D) 0.115 M
E) 0.061 M

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
44
Nitrogen and oxygen gases may react to form nitrogen monoxide. At 1500 °C, Kc equals 1.0 × 10?5. N2(g) + O2(g) 2 NO(g)
If 0.030 mol N2 and 0.030 mol O2 are sealed in a 1.0 L flask at 1500 °C, what is the concentration of NO(g) when equilibrium is established?
A) 3.0 × 10?7 M
B) 4.7 × 10?5 M
C) 9.5 × 10?5 M
D) 3.0 × 10?2 M
E) 9.1 × 101 M
If 0.030 mol N2 and 0.030 mol O2 are sealed in a 1.0 L flask at 1500 °C, what is the concentration of NO(g) when equilibrium is established?
A) 3.0 × 10?7 M
B) 4.7 × 10?5 M
C) 9.5 × 10?5 M
D) 3.0 × 10?2 M
E) 9.1 × 101 M

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
45
For the reaction N2O4(g) 2NO2(g), Kp = 0.148 at a temperature of 298 K. What is Kp for the following reaction? 12NO2(g) 6N2O4(g)
A) 9.52 × 104
B) 0.888
C) 1.05 × 10-5
D) 1.13
E) 6.76
A) 9.52 × 104
B) 0.888
C) 1.05 × 10-5
D) 1.13
E) 6.76

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
46
Consider the following equilibrium:
CO2(g) + H2(g) CO(g) + H2O(g); Kc = 1.6 at 1260 K
Suppose 0.019 mol CO2 and 0.030 mol H2 are placed in a 3.00-L vessel at 1260 K. What is the equilibrium partial pressure of CO(g)? (R = 0.0821 L · atm/K·mol)
A) 4 atm
B) 0.35 atm
C) 1.6 atm
D) 0.66 atm
E) 1 atm
CO2(g) + H2(g) CO(g) + H2O(g); Kc = 1.6 at 1260 K
Suppose 0.019 mol CO2 and 0.030 mol H2 are placed in a 3.00-L vessel at 1260 K. What is the equilibrium partial pressure of CO(g)? (R = 0.0821 L · atm/K·mol)
A) 4 atm
B) 0.35 atm
C) 1.6 atm
D) 0.66 atm
E) 1 atm

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
47
Sulfuryl chloride decomposes to sulfur dioxide and chlorine.
SO2Cl2(g) SO2(g) + Cl2(g)
Kc is 0.045 at 648 K. If an initial concentration of 0.075 M SO2Cl2 is allowed to equilibrate, what is the equilibrium concentration of Cl2?
A) 0.0034 M
B) 0.030 M
C) 0.040 M
D) 0.058 M
E) 0.075 M
SO2Cl2(g) SO2(g) + Cl2(g)
Kc is 0.045 at 648 K. If an initial concentration of 0.075 M SO2Cl2 is allowed to equilibrate, what is the equilibrium concentration of Cl2?
A) 0.0034 M
B) 0.030 M
C) 0.040 M
D) 0.058 M
E) 0.075 M

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
48
At a given temperature, K = 0.024 for the equilibrium:
PCl5(g) PCl3(g) + Cl2(g)
What is K for:
Cl2(g) + PCl3(g) PCl5(g)?
A) 1700
B) 24
C) 0.00058
D) 42
E) 0.024
PCl5(g) PCl3(g) + Cl2(g)
What is K for:
Cl2(g) + PCl3(g) PCl5(g)?
A) 1700
B) 24
C) 0.00058
D) 42
E) 0.024

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
49
At 700 K, Kp for the following equilibrium is
5.6 × 10-3. 2HgO(s) 2Hg(l) + O2(g)
Suppose 43.1 g of mercury(II) oxide is placed in a sealed 4.00-L vessel at 700 K. What is the partial pressure of oxygen gas at equilibrium?
(R = 0.0821 L · atm/(K · mol))
A) 0.074 atm
B) 0.0056 atm
C) 2.8 atm
D) 14 atm
E) 1.4 atm
5.6 × 10-3. 2HgO(s) 2Hg(l) + O2(g)
Suppose 43.1 g of mercury(II) oxide is placed in a sealed 4.00-L vessel at 700 K. What is the partial pressure of oxygen gas at equilibrium?
(R = 0.0821 L · atm/(K · mol))
A) 0.074 atm
B) 0.0056 atm
C) 2.8 atm
D) 14 atm
E) 1.4 atm

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
50
If Kc = 0.124 for A2 + 2B 2AB, what is the value of Kc for the reaction 4AB 2A2 + 4B?
A) 0.124
B) 0.248
C) 65.0
D) -0.124
E) 4.03
A) 0.124
B) 0.248
C) 65.0
D) -0.124
E) 4.03

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
51
The equilibrium constant at 25 °C for the dissolution of silver iodide is
8.5 × 10-17. AgI(s) Ag+(aq) + I-(aq)
If an excess quantity of AgI(s) is added to water and allowed to equilibrate, what is the equilibrium concentration of I-?
A) 7.2 × 10-33 M
B) 4.3 × 10-17 M
C) 8.5 × 10-17 M
D) 6.5 × 10-9 M
E) 9.2 × 10-9 M
8.5 × 10-17. AgI(s) Ag+(aq) + I-(aq)
If an excess quantity of AgI(s) is added to water and allowed to equilibrate, what is the equilibrium concentration of I-?
A) 7.2 × 10-33 M
B) 4.3 × 10-17 M
C) 8.5 × 10-17 M
D) 6.5 × 10-9 M
E) 9.2 × 10-9 M

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
52
For the equilibrium N2O4(g) 2NO2(g), at 298 K, Kp = 0.15. For this reaction system, it is found that the partial pressure of N2O4 is 3.7 × 10-2 atm at equilibrium. What is the partial pressure of NO2 at equilibrium?
A) 4.9 atm
B) 24 atm
C) 0.0016 atm
D) 0.0055 atm
E) 0.074 atm
A) 4.9 atm
B) 24 atm
C) 0.0016 atm
D) 0.0055 atm
E) 0.074 atm

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
53
A mixture of nitrogen and hydrogen was allowed to come to equilibrium at a given temperature. 3H2 + N2 2NH3
An analysis of the mixture at equilibrium revealed 2.1 mol N2, 2.8 mol H2, and 1.8 mol NH3. How many moles of H2 were present at the beginning of the reaction?
A) 2.8
B) 4.2
C) 4.6
D) 5.5
E) 4.0
An analysis of the mixture at equilibrium revealed 2.1 mol N2, 2.8 mol H2, and 1.8 mol NH3. How many moles of H2 were present at the beginning of the reaction?
A) 2.8
B) 4.2
C) 4.6
D) 5.5
E) 4.0

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
54
Consider the reaction H2 + I2 2HI for which Kc = 44.0 at a high temperature. If an equimolar mixture of reactants gives the concentration of the product to be 0.50 M at equilibrium, determine the equilibrium concentration of the hydrogen.
A) 7.5 × 10-2 M
B) 1.1 × 10-1 M
C) 3.8 × 10-2 M
D) 1.3 × 101 M
E) 5.7 × 10-3 M
A) 7.5 × 10-2 M
B) 1.1 × 10-1 M
C) 3.8 × 10-2 M
D) 1.3 × 101 M
E) 5.7 × 10-3 M

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
55
Nitrosyl bromide decomposes according to the chemical equation below. 2 NOBr(g) 2 NO(g) + Br2(g)
When 0.260 atm of NOBr is sealed in a flask and allowed to reach equilibrium, 22% of the NOBr decomposes. What is the equilibrium constant, Kp, for the reaction?
A) 2.3 × 10-3
B) 4.5 × 10-3
C) 3.5 × 10-2
D) 4.8 × 10-2
E) 8.0 × 10-2
When 0.260 atm of NOBr is sealed in a flask and allowed to reach equilibrium, 22% of the NOBr decomposes. What is the equilibrium constant, Kp, for the reaction?
A) 2.3 × 10-3
B) 4.5 × 10-3
C) 3.5 × 10-2
D) 4.8 × 10-2
E) 8.0 × 10-2

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
56
The equilibrium constant (Kc) for the decomposition of ammonium hydrogen sulfide, NH4HS(s) NH3(g) + H2S(g), is 1.8 × 10-4 at 25 °C. If excess NH4HS(s) is allowed to equilibrate at 25 °C, what is the equilibrium concentration of NH3?
A) 3.2 × 10-8 M
B) 9.0 × 10-5 M
C) 1.8 × 10-4 M
D) 6.7 × 10-3 M
E) 1.3 × 10-2 M
A) 3.2 × 10-8 M
B) 9.0 × 10-5 M
C) 1.8 × 10-4 M
D) 6.7 × 10-3 M
E) 1.3 × 10-2 M

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
57
For the equilibrium PCl5(g) PCl3(g) + Cl2(g), Kc = 2.0 × 101 at 240°C. If pure PCl5 is placed in a 1.00-L container and allowed to come to equilibrium, and the equilibrium concentration of PCl3(g) is 0.50 M, what is the equilibrium concentration of PCl5(g)?
A) 1 M
B) 0.25 M
C) 0.025 M
D) 0.012 M
E) 6.3 M
A) 1 M
B) 0.25 M
C) 0.025 M
D) 0.012 M
E) 6.3 M

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
58
At 800 K, the equilibrium constant, Kp, for the following reaction is 3.2 × 10-7. 2 H2S(g) 2 H2(g) + S2(g)
A reaction vessel at 800 K initially contains 3.00 atm of H2S. If the reaction is allowed to equilibrate, what is the equilibrium pressure of S2?
A) 8.5 × 10-5 atm
B) 6.2 × 10-3 atm
C) 9.0 × 10-3 atm
D) 1.1 × 10-2 atm
E) 1.4 × 10-2 atm
A reaction vessel at 800 K initially contains 3.00 atm of H2S. If the reaction is allowed to equilibrate, what is the equilibrium pressure of S2?
A) 8.5 × 10-5 atm
B) 6.2 × 10-3 atm
C) 9.0 × 10-3 atm
D) 1.1 × 10-2 atm
E) 1.4 × 10-2 atm

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
59
At 25 °C, the decomposition of dinitrogen tetraoxide
N2O4(g) 2 NO2(g)
Has an equilibrium constant (Kp) of 0.144. At equilibrium, the total pressure of the system is 0.0758 atm. What is the partial pressure of each gas?
A) 0.0745 atm NO2(g) and 0.0385 N2O4(g)
B) 0.0549 atm NO2(g) and 0.0209 N2O4(g)
C) 0.0531 atm NO2(g) and 0.0227 N2O4(g)
D) 0.0502 atm NO2(g) and 0.0256 N2O4(g)
E) 0.0381 atm NO2(g) and 0.0377 N2O4(g)
N2O4(g) 2 NO2(g)
Has an equilibrium constant (Kp) of 0.144. At equilibrium, the total pressure of the system is 0.0758 atm. What is the partial pressure of each gas?
A) 0.0745 atm NO2(g) and 0.0385 N2O4(g)
B) 0.0549 atm NO2(g) and 0.0209 N2O4(g)
C) 0.0531 atm NO2(g) and 0.0227 N2O4(g)
D) 0.0502 atm NO2(g) and 0.0256 N2O4(g)
E) 0.0381 atm NO2(g) and 0.0377 N2O4(g)

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
60
For the equilibrium PCl5(g) PCl3(g) + Cl2(g), Kc = 4.0 at 228°C. If pure PCl5 is placed in a 1.00-L container and allowed to come to equilibrium, and the equilibrium concentration of PCl5(g) is 0.19 M, what is the equilibrium concentration of PCl3?
A) 0.095 M
B) 0.4 M
C) 0.19 M
D) 0.87 M
E) 0.009 M
A) 0.095 M
B) 0.4 M
C) 0.19 M
D) 0.87 M
E) 0.009 M

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
61
Consider the following equilibria.
PbBr2(s) Pb2+(aq) + 2 Br-(aq)
K1 = 6.6 × 10-6
Pb(OH)2(s) Pb2+(aq) + 2 OH-(aq)
K2 = 1.4 × 10-15
Determine the equilibrium constant, Kc, for the reaction below.
PbBr2(s) + 2 OH-(aq) Pb(OH)2(s) + 2 Br-(aq)
A) 9.2 × 10-21
B) 2.1 × 10-10
C) 6.6 × 10-6
D) 4.7 × 109
E) 1.1 × 1020
PbBr2(s) Pb2+(aq) + 2 Br-(aq)
K1 = 6.6 × 10-6
Pb(OH)2(s) Pb2+(aq) + 2 OH-(aq)
K2 = 1.4 × 10-15
Determine the equilibrium constant, Kc, for the reaction below.
PbBr2(s) + 2 OH-(aq) Pb(OH)2(s) + 2 Br-(aq)
A) 9.2 × 10-21
B) 2.1 × 10-10
C) 6.6 × 10-6
D) 4.7 × 109
E) 1.1 × 1020

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
62
In 1913, the Haber-Bosch process was patented. The product of the Haber-Bosch process is ________.

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
63
The thermochemical equation for the formation of ammonia from elemental nitrogen and hydrogen is as follows. N2(g) + 3 H2(g) 2 NH3(g)
?H = -92.2 kJ
Given a system that is initially at equilibrium, which of the following actions cause the reaction to proceed to the left?
A) adding N2(g)
B) removing NH3(g)
C) adding a catalyst
D) decreasing the temperature
E) removing H2(g)
?H = -92.2 kJ
Given a system that is initially at equilibrium, which of the following actions cause the reaction to proceed to the left?
A) adding N2(g)
B) removing NH3(g)
C) adding a catalyst
D) decreasing the temperature
E) removing H2(g)

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
64
When the pressure of an equilibrium mixture of SO2, O2, and SO3 is doubled at constant temperature, what the effect on Kp?
2SO2(g) + O2(g) 2SO3(g)
A) Kp is halved.
B) Kp is doubled.
C) Kp is unchanged.
D) Kp is tripled.
E) Kp is decreased by a third.
2SO2(g) + O2(g) 2SO3(g)
A) Kp is halved.
B) Kp is doubled.
C) Kp is unchanged.
D) Kp is tripled.
E) Kp is decreased by a third.

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
65
Which of the following equilibria would not be affected by pressure change at constant temperature?
A) CO2(g) + H2(g) CO(g) + H2O(g)
B) CO(g) + O2(g) CO2(g)
C) 2 Hg( ) + O2(g) 2 HgO(s)
D) 2 H2(g) + O2(g) 2 H2O( )
E) CaCO3(s) CaO(s) + CO2(g)
A) CO2(g) + H2(g) CO(g) + H2O(g)
B) CO(g) + O2(g) CO2(g)
C) 2 Hg( ) + O2(g) 2 HgO(s)
D) 2 H2(g) + O2(g) 2 H2O( )
E) CaCO3(s) CaO(s) + CO2(g)

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
66
The standard enthalpy of formation of ammonia is -46.1 kJ/mol.
1/2 N2(g) + 3/2 H2(g)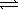 NH3(g)
NH3(g)
Commercially, the reaction is carried out at high temperatures. Using your knowledge of kinetics and equilibrium, explain an advantage and a disadvantage of synthesizing ammonia at high temperatures.
1/2 N2(g) + 3/2 H2(g)
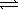 NH3(g)
NH3(g)Commercially, the reaction is carried out at high temperatures. Using your knowledge of kinetics and equilibrium, explain an advantage and a disadvantage of synthesizing ammonia at high temperatures.

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
67
In which of the following reactions does a decrease in the volume of the reaction vessel at constant temperature favor the formation of the products?
A) 2 H2(g) + O2(g) 2 H2O(g)
B) NO2(g) + CO(g) NO(g) + CO2(g)
C) H2(g) + I2(g) 2 HI(g)
D) 2 O3(g) 3 O2(g)
E) MgCO3(s) MgO(s) + CO2(g)
A) 2 H2(g) + O2(g) 2 H2O(g)
B) NO2(g) + CO(g) NO(g) + CO2(g)
C) H2(g) + I2(g) 2 HI(g)
D) 2 O3(g) 3 O2(g)
E) MgCO3(s) MgO(s) + CO2(g)

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
68
When 1.0 mole of acetic acid is diluted with water to a volume of 1.0 L at 25 °C, 0.42% of the acetic acid ionizes to form acetate ion and hydronium ion.
CH3CO2H(aq) + H2O(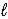 )
) 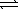 CH3CO2-(aq) + H3O+(aq)
CH3CO2-(aq) + H3O+(aq)
What percentage of the acid ionizes when 0.75 mole of acetic acid is diluted with water to 1.0 L at 25 °C?
CH3CO2H(aq) + H2O(
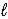 )
) 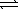 CH3CO2-(aq) + H3O+(aq)
CH3CO2-(aq) + H3O+(aq)What percentage of the acid ionizes when 0.75 mole of acetic acid is diluted with water to 1.0 L at 25 °C?

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
69
Given the following equilibria,
Ni2+(aq) + 2 OH-(aq) Ni(OH)2(s)
K1 = 1.8 × 1015
Ni2+(aq) + 4 CN-(aq) Ni(CN)42-(aq)
K2 = 2.0 × 1031
Determine the equilibrium constant, Kc, for the following reaction.
Ni(OH)2(s) + 4 CN-(aq) Ni(CN)42-(aq) + 2 OH-(aq)
A) 2.8 × 10-47
B) 9.0 × 10-17
C) 1.8 × 1015
D) 1.1 × 1016
E) 3.6 × 1046
Ni2+(aq) + 2 OH-(aq) Ni(OH)2(s)
K1 = 1.8 × 1015
Ni2+(aq) + 4 CN-(aq) Ni(CN)42-(aq)
K2 = 2.0 × 1031
Determine the equilibrium constant, Kc, for the following reaction.
Ni(OH)2(s) + 4 CN-(aq) Ni(CN)42-(aq) + 2 OH-(aq)
A) 2.8 × 10-47
B) 9.0 × 10-17
C) 1.8 × 1015
D) 1.1 × 1016
E) 3.6 × 1046

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
70
Consider the following equilibrium. PCl3(g) + Cl2(g) PCl5(g) ?H = -92 kJ
The concentration of PCl3 at equilibrium can be increased by:
A) decreasing the temperature.
B) adding Cl2 to the system.
C) adding PCl5 to the system.
D) increasing the pressure.
E) adding a catalyst.
The concentration of PCl3 at equilibrium can be increased by:
A) decreasing the temperature.
B) adding Cl2 to the system.
C) adding PCl5 to the system.
D) increasing the pressure.
E) adding a catalyst.

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
71
Which of the following statements is true if reaction quotient (Q) is greater than equilibrium constant (K)?
A) Reactant concentrations will increase.
B) Product concentrations will increase.
C) Reactants will convert to products.
D) Standard enthalpy of formation of products decreases.
E) Standard enthalpy of formation of reactants increases.
A) Reactant concentrations will increase.
B) Product concentrations will increase.
C) Reactants will convert to products.
D) Standard enthalpy of formation of products decreases.
E) Standard enthalpy of formation of reactants increases.

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
72
If a stress is applied to an equilibrium system, the system will respond in such a way as to relieve that stress. This is a statement of ________ principle.

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
73
Given the equilibrium constants for the equilibria, NH4+(aq) + H2O(l) NH3(aq) + H3O+(aq); Kc = 2H2O(l) 2H3O+(aq); Kc = determine Kc for the following equilibrium.
CH3COOH(aq) + NH3(aq) CH3COO?(aq) + NH4+(aq)
A) 3.08 × 104
B) 3.25 × 10-5
C) 9.96 × 10-15
D) 1.00 × 1014
E) 1.75 × 10-5
CH3COOH(aq) + NH3(aq) CH3COO?(aq) + NH4+(aq)
A) 3.08 × 104
B) 3.25 × 10-5
C) 9.96 × 10-15
D) 1.00 × 1014
E) 1.75 × 10-5

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
74
The symbol Q is called the ________.

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
75
Assume that the following chemical reaction is at equilibrium.
2 ICl(g) I2(g) + Cl2(g)
?H° = +26.9 kJ
At 25 °C, Kp = 2.0 × 105. If the temperature is increase to 45 °C, which statement applies?
A) Kp will decrease and the reaction will proceed in the backward direction.
B) Kp will decrease and the reaction will proceed in the forward direction.
C) Kp will remain unchanged and the reaction will proceed in the forward direction.
D) Kp will increase and the reaction will proceed in the backward direction.
E) Kp will increase and the reaction will proceed in the forward direction.
2 ICl(g) I2(g) + Cl2(g)
?H° = +26.9 kJ
At 25 °C, Kp = 2.0 × 105. If the temperature is increase to 45 °C, which statement applies?
A) Kp will decrease and the reaction will proceed in the backward direction.
B) Kp will decrease and the reaction will proceed in the forward direction.
C) Kp will remain unchanged and the reaction will proceed in the forward direction.
D) Kp will increase and the reaction will proceed in the backward direction.
E) Kp will increase and the reaction will proceed in the forward direction.

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck



