Deck 11: The Unsaturated Hydrocarbons: Alkenes, alkynes, and Aromatics
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/74
Play
Full screen (f)
Deck 11: The Unsaturated Hydrocarbons: Alkenes, alkynes, and Aromatics
1
What is the relationship between the two compounds shown below? 
A)They are identical.
B)They are geometric isomers.
C)They are constitutional isomers.
D)They are conformational isomers.
E)They are not related.

A)They are identical.
B)They are geometric isomers.
C)They are constitutional isomers.
D)They are conformational isomers.
E)They are not related.
They are geometric isomers.
2
Which of the following best describes the changes that occurred in the reaction shown below? 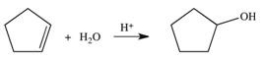
A)An H atom and an -OH group have been added to the reactant.
B)An -OH group has been added to the reactant.
C)An -OH group has replaced an H atom on the ring.
D)Two H atoms have been added.
E)An -OH group has replaced a carbon atom on the ring.

A)An H atom and an -OH group have been added to the reactant.
B)An -OH group has been added to the reactant.
C)An -OH group has replaced an H atom on the ring.
D)Two H atoms have been added.
E)An -OH group has replaced a carbon atom on the ring.
An H atom and an -OH group have been added to the reactant.
3
Polyvinyl chloride (PVC)is formed by addition polymerization of vinyl chloride,CH2=CHCl.Which of the following depicts a representative section of the PVC structure?
A)
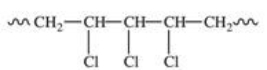
B)
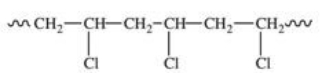
C)
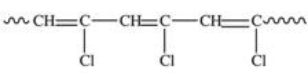
D)
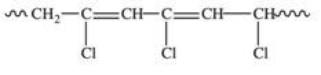
E)
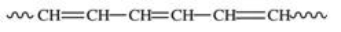
A)
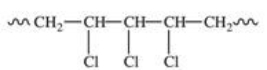
B)

C)

D)

E)
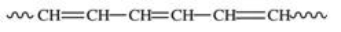

4
What is the name of the major product in the reaction shown below? 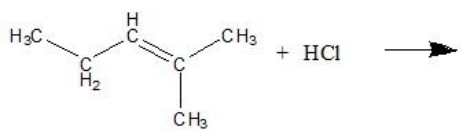
A)2-methyl-2-pentene
B)2-chloro-2-methyl-2-pentene
C)3-chloro-2-methylpentane
D)2-methylpentane
E)2-chloro-2-methylpentane

A)2-methyl-2-pentene
B)2-chloro-2-methyl-2-pentene
C)3-chloro-2-methylpentane
D)2-methylpentane
E)2-chloro-2-methylpentane

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
5
An organic compound is classified as aromatic if it fits which of the following criteria?
A)It is fragrant.
B)It is cyclic.
C)It contains at least one C=C bond.
D)It contains a benzene ring.
E)It follows Markovnikov's rule.
A)It is fragrant.
B)It is cyclic.
C)It contains at least one C=C bond.
D)It contains a benzene ring.
E)It follows Markovnikov's rule.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
6
What is the IUPAC name of HC≡CH?
A)acetyne
B)butyne
C)ethyne
D)diyne
E)triyne
A)acetyne
B)butyne
C)ethyne
D)diyne
E)triyne

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
7
Bromine (Br2)is often used as a quick test for the presence of unsaturation in an aliphatic hydrocarbon.Bromine in CCl4 is red.When bromine reacts with an alkene or an alkyne,the alkyl halide formed is colorless; hence the disappearance of the red color is a positive test for unsaturation.A student tested the contents of two vials,A and B,both containing compounds with the molecular formula C6H12.Vial A decolorized bromine,but vial B did not.How may the results for vial B be interpreted?
A)Vial B must contain a compound with only C−C single bonds.
B)Vial B must contain a compound with a triple bond.
C)Vial B must contain a compound with more than one C=C bond.
D)Vial B must contain a cyclic compound.
E)Vial B must contain a compound that is not a hydrocarbon.
A)Vial B must contain a compound with only C−C single bonds.
B)Vial B must contain a compound with a triple bond.
C)Vial B must contain a compound with more than one C=C bond.
D)Vial B must contain a cyclic compound.
E)Vial B must contain a compound that is not a hydrocarbon.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
8
What is true about a compound with the name 3,3-dimethyl-1-butyne?
A)It is aromatic.
B)It contains a carbonyl group.
C)It contains a carbon-carbon double bond.
D)It contains a carbon-carbon triple bond.
E)It contains a benzene ring.
A)It is aromatic.
B)It contains a carbonyl group.
C)It contains a carbon-carbon double bond.
D)It contains a carbon-carbon triple bond.
E)It contains a benzene ring.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
9
Which statement concerning the structure of the compound 1-ethylcyclohexene is FALSE?
A)It contains a ring of six carbons.
B)It contains one C=C.
C)It contains a two-carbon alkyl substituent on the parent chain.
D)It is an alkene.
E)All of these are correct.
A)It contains a ring of six carbons.
B)It contains one C=C.
C)It contains a two-carbon alkyl substituent on the parent chain.
D)It is an alkene.
E)All of these are correct.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
10
What is the IUPAC name of the compound shown below?
A)heptyne
B)butyl methyl ethene
C)1-methyl-2-butyl ethyne
D)2-hexene
E)2-heptyne
A)heptyne
B)butyl methyl ethene
C)1-methyl-2-butyl ethyne
D)2-hexene
E)2-heptyne

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
11
Which compound shown below is NOT an isomer of the molecular formula C5H8?
A)
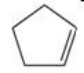
B)
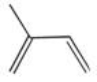
C)

D)

E)
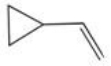
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
12
What is the molecular formula of the structure shown below? 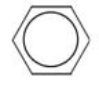
A)C6
B)C6H6
C)C6H10
D)C6H12
E)C6H14

A)C6
B)C6H6
C)C6H10
D)C6H12
E)C6H14

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
13
What is the product of the reaction shown below? 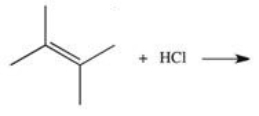
A)
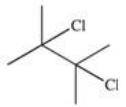
B)
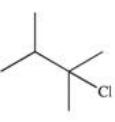
C)
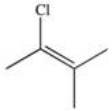
D)
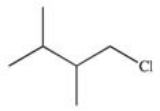
E)

A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
14
Which of the following correctly states Markovnikov's rule as it applies to the addition of the generic reagent HX to an alkene?
A)The hydrogen atom of the HX reagent adds to the carbon atom of the double bond that has more alkyl groups attached to it.
B)The hydrogen atom of the HX reagent adds to the carbon atom of the double bond that doesn't have any hydrogen atoms attached to it.
C)The hydrogen atom of the HX reagent adds to the carbon atom of the double bond that has more hydrogen atoms attached to it.
D)The X atom of the HX reagent adds to the carbon atom of the double bond that has more halogen atoms attached to it.
E)The X atom of the HX reagent adds to the carbon of the double bond that is closer to the end of the carbon chain.
A)The hydrogen atom of the HX reagent adds to the carbon atom of the double bond that has more alkyl groups attached to it.
B)The hydrogen atom of the HX reagent adds to the carbon atom of the double bond that doesn't have any hydrogen atoms attached to it.
C)The hydrogen atom of the HX reagent adds to the carbon atom of the double bond that has more hydrogen atoms attached to it.
D)The X atom of the HX reagent adds to the carbon atom of the double bond that has more halogen atoms attached to it.
E)The X atom of the HX reagent adds to the carbon of the double bond that is closer to the end of the carbon chain.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
15
In the food industry,what substance undergoes hydrogenation to produce margarine?
A)vegetable oils
B)cholesterol
C)wheat
D)eggs
E)lard
A)vegetable oils
B)cholesterol
C)wheat
D)eggs
E)lard

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
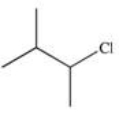
16
Which of the following reactions is best suited to prepare the compound 2,2-dibromopentane?
A)

B)
C)
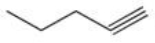
D)
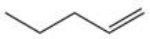
E)
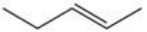
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
17
What is the product of the following reaction?
A)
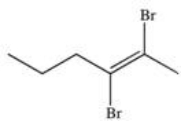
B)
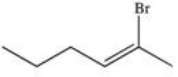
C)
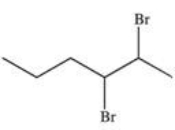
D)
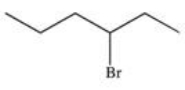
E)
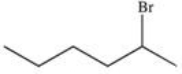
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
18
Which of the following compounds exists as cis-trans isomers?
A)1-pentene
B)butane
C)2-methyl-1-butene
D)3-hexene
E)2,3-dimethyl-2-butene
A)1-pentene
B)butane
C)2-methyl-1-butene
D)3-hexene
E)2,3-dimethyl-2-butene

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
19
Which term correctly describes the substitution pattern on the benzene ring in Fenfluramine,one component of the appetite suppressant Fen-Phen? 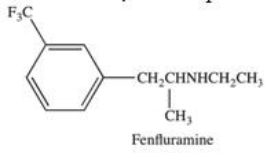
A)trans
B)syn
C)meta
D)cis
E)ortho

A)trans
B)syn
C)meta
D)cis
E)ortho

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
20
Which structure correctly represents the major product of the following reaction? 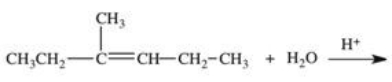
A)
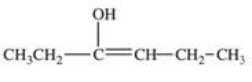
B)
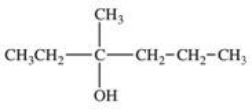
C)
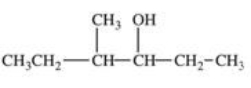
D)
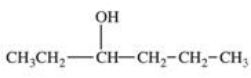
E)
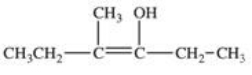
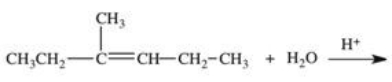
A)

B)

C)

D)

E)
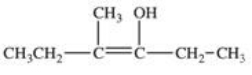

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
21
What is the structure and name of the product of the following reaction?
A)CH3CH2CHCH3,butane
B)CH3CH2CH2CH3,butane
C)CH3CH2C?CH,butyne
D)CH3CH2CH2CH3,propane
E)None of the choices are correct.
A)CH3CH2CHCH3,butane
B)CH3CH2CH2CH3,butane
C)CH3CH2C?CH,butyne
D)CH3CH2CH2CH3,propane
E)None of the choices are correct.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
22
Sunscreens absorb UV radiation and protect the skin from its harmful effects.Commercially available sunscreens contain a benzene ring in the structure of the active ingredient.Which of the following compounds might be effective as an active ingredient in a commercial sunscreen?
A)
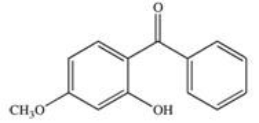
B)
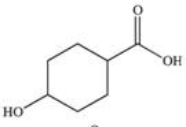
C)
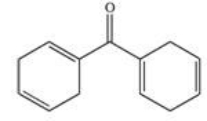
D)
E)All of the compounds would be effective.
A)

B)

C)

D)

E)All of the compounds would be effective.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
23
What catalysts are often used in the hydrogenation of alkenes?
A)platinum,palladium,and nickel
B)copper,chromium,and zinc
C)acid,base,and nickel
D)gold,silver,and aluminum
E)platinum,gold,and silver
A)platinum,palladium,and nickel
B)copper,chromium,and zinc
C)acid,base,and nickel
D)gold,silver,and aluminum
E)platinum,gold,and silver

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
24
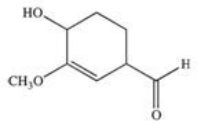
What is the IUPAC name of the following compound? 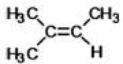
A)1,1,2-trimethylethene
B)2-methylbuta-2-ene
C)1,2-dimethylpropene
D)2-methyl-2-butene
E)2,3-dimethylbutene

A)1,1,2-trimethylethene
B)2-methylbuta-2-ene
C)1,2-dimethylpropene
D)2-methyl-2-butene
E)2,3-dimethylbutene

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
25
Provide the IUPAC name of the following compound: 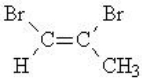
A)cis-1,2-dibromopropane
B)trans-1,2-dibromo-1-methylethene
C)cis-1,2-dibromo-2-methylethene
D)cis-1,2-dibromopropene
E)trans-1,2-dibromopropene

A)cis-1,2-dibromopropane
B)trans-1,2-dibromo-1-methylethene
C)cis-1,2-dibromo-2-methylethene
D)cis-1,2-dibromopropene
E)trans-1,2-dibromopropene

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
26
What is the structure of the product of the following reaction?
A)CH3CH2O
B)CH3CH3
C)CH3CH2OH
D)HOCH2CH2OH
E)None of the choices are correct.
A)CH3CH2O
B)CH3CH3
C)CH3CH2OH
D)HOCH2CH2OH
E)None of the choices are correct.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
27
The compound ortho-dichlorobenzene has substituents on which carbon atoms of the benzene ring?
A)C1 and C2
B)C1 and C3
C)C1 and C4
D)C1 and C 5
E)C1 only
A)C1 and C2
B)C1 and C3
C)C1 and C4
D)C1 and C 5
E)C1 only

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
28
When 4,4-dimethyl-2-pentene adds H2 in an addition reaction,what is the name of the product that results?
A)pentane
B)2,2-dimethylpentane
C)4,4-dimethylpentane
D)4,4-dimethyl-2-pentane
E)2,3-dihydro-4,4-dimethylpentane
A)pentane
B)2,2-dimethylpentane
C)4,4-dimethylpentane
D)4,4-dimethyl-2-pentane
E)2,3-dihydro-4,4-dimethylpentane

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
29
What is the structure and name of the major product of the following reaction? CH3CH=CH2 + HCl → ?
A)CH3CH2CH2Cl,3-chloropropane
B)CH3CH2CH2Cl,1-chloropropane
C)CH2CHClCH3,2-chloropropane
D)CH3CH=CHCl,1-chloro-1-propene
E)There is no major product.
A)CH3CH2CH2Cl,3-chloropropane
B)CH3CH2CH2Cl,1-chloropropane
C)CH2CHClCH3,2-chloropropane
D)CH3CH=CHCl,1-chloro-1-propene
E)There is no major product.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
30
How many moles of hydrogen gas (H2)are needed to convert one mole of ethyne to ethane?
A)0.5
B)1
C)2
D)3
E)8
A)0.5
B)1
C)2
D)3
E)8

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
31
Which of the following reactions would provide the highest yield of 3-bromohexane?
A)

B)

C)
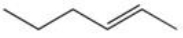
D)

E)All would provide equal yields of 3-bromohexane.
A)

B)

C)

D)

E)All would provide equal yields of 3-bromohexane.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
32
Which of the following terms best describes the geometry around a carbon atom with the bonding pattern shown? 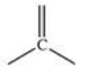
A)tetrahedral
B)straight
C)saturated
D)trigonal planar
E)cyclic

A)tetrahedral
B)straight
C)saturated
D)trigonal planar
E)cyclic

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
33
What is the geometry and bond angle around a carbon atom that has one triple bond and one single bond to its neighboring atoms?
A)linear,180o
B)bent,120o
C)trigonal planar,120o
D)trigonal,180o
E)tetrahedral,109.5o
A)linear,180o
B)bent,120o
C)trigonal planar,120o
D)trigonal,180o
E)tetrahedral,109.5o

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
34
What is the molecular formula of the simplest alkyne?
A)C2H4
B)CH4
C)C4H8
D)C2H2
E)C4H6
A)C2H4
B)CH4
C)C4H8
D)C2H2
E)C4H6

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
35
What is the IUPAC name of the following compound? 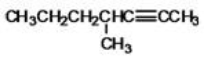
A)4-methyl-2-heptyne
B)4-methyl-5-heptyne
C)4-ethyl-4-methylbutane
D)4-propyl-2-pentyne
E)2-propyl-3-butyne
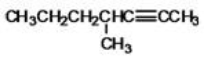
A)4-methyl-2-heptyne
B)4-methyl-5-heptyne
C)4-ethyl-4-methylbutane
D)4-propyl-2-pentyne
E)2-propyl-3-butyne

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
36
Which of the following is the correct condensed formula for 2-butyne?
A)CH≡CCH2CH3
B)CH3C≡CCH3
C)CH3CH=CHCH3
D)CH3CH2≡CH2CH3
E)None of the choices are correct.
A)CH≡CCH2CH3
B)CH3C≡CCH3
C)CH3CH=CHCH3
D)CH3CH2≡CH2CH3
E)None of the choices are correct.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
37
What is the IUPAC name of the following compound? 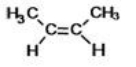
A)cis-2-butene
B)trans-2-butene
C)cis-1,2-dimethylethene
D)trans-1,2-dimethylethene
E)cis-1,2-dimethylbutene

A)cis-2-butene
B)trans-2-butene
C)cis-1,2-dimethylethene
D)trans-1,2-dimethylethene
E)cis-1,2-dimethylbutene

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
38
What is the common name of the compound shown? 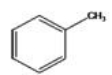
A)methylbenzene
B)toluene
C)methyltoluene
D)methylcyclohexene
E)None of the choices are correct.

A)methylbenzene
B)toluene
C)methyltoluene
D)methylcyclohexene
E)None of the choices are correct.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
39
What is the proper IUPAC name for an alkene that contains three carbon atoms?
A)1-methylethene
B)2-methylethene
C)propene
D)propane
E)1-propyne
A)1-methylethene
B)2-methylethene
C)propene
D)propane
E)1-propyne

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
40
How many moles of hydrogen gas (H2)are needed to convert one mole of ethene to ethane?
A)1
B)2
C)3
D)4
E)8
A)1
B)2
C)3
D)4
E)8

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
41
Which of the following is the correct molecular formula for cyclopentene?
A)C4H8
B)C5H8
C)C5H12
D)C5H10
E)C5H6
A)C4H8
B)C5H8
C)C5H12
D)C5H10
E)C5H6

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
42
para-Chlorotoluene is the name of an organic compound.Based on its name,what information about the structure of this compound is FALSE?
A)It is an alkene.
B)The structure contains a benzene ring.
C)A methyl group and a chlorine atom are present as substituents.
D)The substituents that are present have a 1,4 relationship.
E)All of these are correct.
A)It is an alkene.
B)The structure contains a benzene ring.
C)A methyl group and a chlorine atom are present as substituents.
D)The substituents that are present have a 1,4 relationship.
E)All of these are correct.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
43
What is the proper name of the product formed by the hydrogenation of 2-octene?
A)2-octane
B)1-octene
C)2-octyne
D)octane
E)cyclooctane
A)2-octane
B)1-octene
C)2-octyne
D)octane
E)cyclooctane

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
44
What is the correct IUPAC name for the compound shown below? 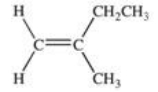
A)2-methyl-1-butene
B)2-ethylpropene
C)cis-2-methyl-1-butene
D)trans-2-ethyl-2-methylbutene
E)trans-2-ethyl-2-methylethene

A)2-methyl-1-butene
B)2-ethylpropene
C)cis-2-methyl-1-butene
D)trans-2-ethyl-2-methylbutene
E)trans-2-ethyl-2-methylethene

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
45
What is the IUPAC name of the following compound?
A)propene
B)1-methylethyne
C)propyne
D)acetylene
E)2-methylethyne
A)propene
B)1-methylethyne
C)propyne
D)acetylene
E)2-methylethyne

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
46
Which of the following reaction types is most characteristic of aromatic compounds?
A)substitution
B)addition
C)elimination
D)combination
E)decomposition
A)substitution
B)addition
C)elimination
D)combination
E)decomposition

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
47
Which reaction type is most characteristic of alkenes?
A)substitution
B)addition
C)elimination
D)combination
E)decomposition
A)substitution
B)addition
C)elimination
D)combination
E)decomposition

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
48
What product results when benzene undergoes the halogenation reaction below? 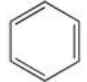
A)
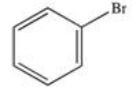
B)
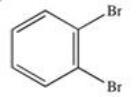
C)
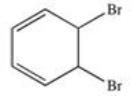
D)
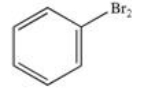
E)There is no reaction; benzene is unreactive toward bromine.

A)

B)

C)

D)

E)There is no reaction; benzene is unreactive toward bromine.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
49
What are the health risks associated with trans-fatty acids?
A)elevated levels of LDL (bad)cholesterol
B)decreased levels of HDL (good)cholesterol
C)increased risk of developing type-2 diabetes
D)All of the choices are correct.
E)None of the choices are correct.
A)elevated levels of LDL (bad)cholesterol
B)decreased levels of HDL (good)cholesterol
C)increased risk of developing type-2 diabetes
D)All of the choices are correct.
E)None of the choices are correct.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
50
What is the proper IUPAC name of the following compound? 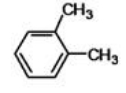
A)toluene
B)phenol
C)para-dimethylbenzene
D)ortho-toluene
E)1,2-dimethylbenzene

A)toluene
B)phenol
C)para-dimethylbenzene
D)ortho-toluene
E)1,2-dimethylbenzene

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
51
What is the IUPAC name of the simplest alkene?
A)cyclopropene
B)methene
C)propyne
D)ethylene
E)ethene
A)cyclopropene
B)methene
C)propyne
D)ethylene
E)ethene

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
52
The polymer shown was formed by the addition polymerization of an alkene. 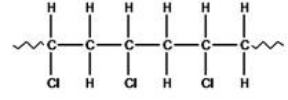 What is the molecular formula of the alkene that formed the polymer?
What is the molecular formula of the alkene that formed the polymer?
A)C2H2Cl2
B)C2H3Cl
C)C3H5Cl
D)C3H7Cl
E)C4H6Cl2
 What is the molecular formula of the alkene that formed the polymer?
What is the molecular formula of the alkene that formed the polymer?A)C2H2Cl2
B)C2H3Cl
C)C3H5Cl
D)C3H7Cl
E)C4H6Cl2

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
53
Which of the following types of compounds is produced by the hydration of an alkene?
A)alkyne
B)phenol
C)alcohol
D)aldehyde
E)ketone
A)alkyne
B)phenol
C)alcohol
D)aldehyde
E)ketone

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
54
What type of product is formed in the reaction between an alkene and a hydrogen halide?
A)alcohol
B)alkyl halide
C)triol
D)aldehyde
E)ketone
A)alcohol
B)alkyl halide
C)triol
D)aldehyde
E)ketone

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
55
What are the bond angles around the carbon atoms in CH2=CH2?
A)180°
B)150°
C)120°
D)109.5°
E)90°
A)180°
B)150°
C)120°
D)109.5°
E)90°

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
56
What is the IUPAC name of the following compound? 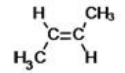
A)1,2-dimethylbutene
B)cis-2-butene
C)trans-2-butene
D)1,2-dimethylethene
E)cis-1,2-dimethylbutene

A)1,2-dimethylbutene
B)cis-2-butene
C)trans-2-butene
D)1,2-dimethylethene
E)cis-1,2-dimethylbutene

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
57
Bombykol is a pheromone secreted by the female silkworm moth to attract males.This compound contains two alkene double bonds.Which of the following correctly describes the geometry around each? 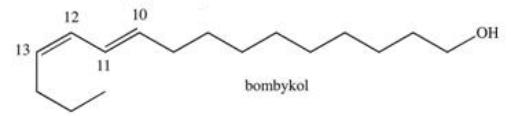
A)The double bond at C10 is cis and the double bond at C12 is trans.
B)The double bonds at C10 and C12 are both cis.
C)The double bonds at C10 and C12 are both trans.
D)The double bond at C10 is trans and the double bond at C12 is cis.
E)It is impossible to tell from the line formula alone.

A)The double bond at C10 is cis and the double bond at C12 is trans.
B)The double bonds at C10 and C12 are both cis.
C)The double bonds at C10 and C12 are both trans.
D)The double bond at C10 is trans and the double bond at C12 is cis.
E)It is impossible to tell from the line formula alone.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
58
What is the IUPAC name of the compound below? 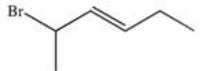
A)trans-1-bromo-1-methyl-2-pentene
B)2-bromohexene
C)trans-2-bromo-3-hexene
D)cis-5-bromo-3-hexene
E)cis-1-bromomethyl-2-pentene

A)trans-1-bromo-1-methyl-2-pentene
B)2-bromohexene
C)trans-2-bromo-3-hexene
D)cis-5-bromo-3-hexene
E)cis-1-bromomethyl-2-pentene

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
59
Which reaction may be used to convert an alkene to an alkane?
A)hydrohalogenation
B)substitution
C)hydrogenation
D)hydration
E)hydrocarbonation
A)hydrohalogenation
B)substitution
C)hydrogenation
D)hydration
E)hydrocarbonation

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
60
Acetylene (ethyne)is a gas at room temperature because its boiling point is -84°C.What type of intermolecular forces hold molecules of acetylene together in a collection?
A)London dispersion forces
B)hydrogen bonding
C)ionic bonding
D)dipole-dipole forces
E)covalent bonds
A)London dispersion forces
B)hydrogen bonding
C)ionic bonding
D)dipole-dipole forces
E)covalent bonds

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
61
Margarine is made by the hydrogenation of vegetable oils.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
62
What is the name of the compound formed by the chlorination (addition of Cl2)of propene?
A)1,1-dichloropropane
B)1,2-dichloropropane
C)2,2-dichloropropane
D)1-chloropropane
E)2-chloropropane
A)1,1-dichloropropane
B)1,2-dichloropropane
C)2,2-dichloropropane
D)1-chloropropane
E)2-chloropropane

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
63
The names ortho-dimethylbenzene and 1,2-dimethylbenzene represent the same compound.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
64
Aromatic compounds undergo addition reactions more readily than substitution reactions because they are extremely stable compounds.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
65
The molecular formula of 3-octene is C8H16.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
66
Hydration of an alkene produces a ketone.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
67
Which of the following correctly match the IUPAC and common system for naming a benzene with two groups attached?
A)1,2-dichlorobenzene and meta-dichlorobenzene
B)2-bromotoluene and ortho-bromotoluene
C)1-bromo-2-chlorobenzene,para-bromochlorobenzene
D)5-chlorotoluene,meta-chlorotoluene
E)2-bromotoluene and ortho-bromotoluene and 1-bromo-2-chlorobenzene,para-bromochlorobenzene
A)1,2-dichlorobenzene and meta-dichlorobenzene
B)2-bromotoluene and ortho-bromotoluene
C)1-bromo-2-chlorobenzene,para-bromochlorobenzene
D)5-chlorotoluene,meta-chlorotoluene
E)2-bromotoluene and ortho-bromotoluene and 1-bromo-2-chlorobenzene,para-bromochlorobenzene

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
68
Palladium is a catalyst used in hydrogenation.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
69
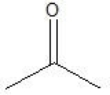
What is the major product formed by the hydration of propene?
A)
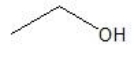
B)

C)
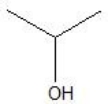
D)
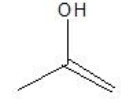
E)
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
70
Both 1-butene and 2-butene have cis- and trans-isomers.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
71
Pyridine,shown below,is a heterocyclic aromatic compound. 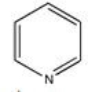


Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
72
What is the name of the organic product in the reaction of benzene and chlorine (with FeCl3 as a catalyst)?
A)2-chlrorobenzene
B)meta-dichlorobenzene
C)1,2-dichlorobenzene
D)1-chlorobenzene
E)chlorobenzene
A)2-chlrorobenzene
B)meta-dichlorobenzene
C)1,2-dichlorobenzene
D)1-chlorobenzene
E)chlorobenzene

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
73
Alkenes contain only double bonds.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
74
The common name of ethyne is acetylene.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck



