Deck 12: Spectroscopy and Structure Determination
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/48
Play
Full screen (f)
Deck 12: Spectroscopy and Structure Determination
1
Bending vibrations in the infared region occur at:
A) 3000 cm-1
B) 2200 cm-1
C) 1700 cm-1
D) below 1400 cm-1
E) over 3000 cm-1
A) 3000 cm-1
B) 2200 cm-1
C) 1700 cm-1
D) below 1400 cm-1
E) over 3000 cm-1
below 1400 cm-1
2
The area in the infared spectrum between 1400 and 400 cm-1 is called the .
A) solid region
B) fingerprint region
C) functional group region
D) location region
E) none of these
A) solid region
B) fingerprint region
C) functional group region
D) location region
E) none of these
fingerprint region
3
Which bond would show a sharp absorption in the IR between 3000 and 2800 cm-1?
A) O-H
B) N-H
C) C=O
D) C-H
E) C-O
A) O-H
B) N-H
C) C=O
D) C-H
E) C-O
C-H
4
An IR spectrum of an unknown organic molecule having the formula C3H6O2 reveals strong absorptions at 2500-3400 cm-1, 1715 cm-1, and 1230 cm-1.To what class of compounds does the unknown belong?
A) alcohol
B) carboxylic acid
C) aldehyde
D) ester
E) ether
A) alcohol
B) carboxylic acid
C) aldehyde
D) ester
E) ether

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
5
As the wavelength of the radiation increases, the _______ decreases.
A) energy
B) frequency
C) wave number
D) all of these
E) all increase
A) energy
B) frequency
C) wave number
D) all of these
E) all increase

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
6
An IR spectrum of an unknown organic molecule having the molecular formula of C4H8O shows no absorptions at 1630-1780 cm-1 or at 3200-3500 cm-1.To what class of compounds does the unknown belong?
A) alcohol
B) carboxylic acid
C) ketone
D) ether
E) aldehyde
A) alcohol
B) carboxylic acid
C) ketone
D) ether
E) aldehyde

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
7
How many peak(s) would you expect to see in the 1H NMR spectrum of 1-bromobutane?
A) 1
B) 2
C) 3
D) 4
E) more than 4
A) 1
B) 2
C) 3
D) 4
E) more than 4

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
8
As the wavelength of the radiation decreases, the increases.
A) energy
B) frequency
C) wave number
D) all of these
E) all decrease
A) energy
B) frequency
C) wave number
D) all of these
E) all decrease

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
9
An IR spectrum of an unknown organic molecule having the formula C3H6O reveals a strong absorption at 1230 cm-1 and has no absorption over 3000 cm-1.To what class of compounds does the unknown belong?
A) alcohol
B) amine
C) aldehyde
D) alkyne
E) ether
A) alcohol
B) amine
C) aldehyde
D) alkyne
E) ether

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
10
Which of the following forms of electromagnetic radiation has the highest energy?
A) radiowaves
B) infrared
C) visible
D) uv
E) x-ray
A) radiowaves
B) infrared
C) visible
D) uv
E) x-ray

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
11
Which of the following compounds will not have a broad band in the 3000 to 3600 cm-1 region of its IR spectrum?
A)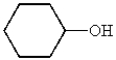
B)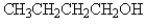
C)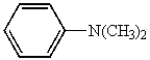
D)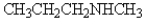
E)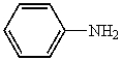
A)

B)
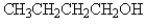
C)

D)
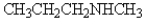
E)


Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
12
How many peaks would you expect to see in the 1H NMR spectrum for the following compound: 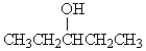
A) 1
B) 2
C) 3
D) 4
E) more than 4
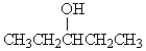
A) 1
B) 2
C) 3
D) 4
E) more than 4

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
13
Which bond would show a strong, broad absorption in the IR between 3600 and 3200 cm-1?
A) O-H
B) C-Cl
C) C=O
D) C-H
E) C-O
A) O-H
B) C-Cl
C) C=O
D) C-H
E) C-O

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
14
Which of the following forms of electromagnetic radiation has the longest wavelength?
A) radiowaves
B) infrared
C) visible
D) uv
E) x-ray
A) radiowaves
B) infrared
C) visible
D) uv
E) x-ray

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
15
Which bond would show a strong, sharp absorption in the IR between 1200 and 1000 cm-1?
A) O-H
B) N-H
C) C=O
D) C-H
E) C-O
A) O-H
B) N-H
C) C=O
D) C-H
E) C-O

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
16
Which bond would show a strong, sharp absorption in the IR between 1800 and 1700 cm-1?
A) O-H
B) N-H
C) C=O
D) C-H
E) C-O
A) O-H
B) N-H
C) C=O
D) C-H
E) C-O

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
17
An IR spectrum of an unknown organic molecule having the formula C3H6O reveals a strong absorption at 1725 cm-1.To what class of compounds does the unknown belong?
A) acid chloride
B) alkene
C) ketone
D) alkyne
E) phenol
A) acid chloride
B) alkene
C) ketone
D) alkyne
E) phenol

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
18
An IR spectrum of an unknown organic molecule having the formula C2H4O reveals a strong absorption at 1715-1725 cm-1.To what class of compounds does the unknown belong?
A) alcohol
B) amine
C) aldehyde
D) alkyne
E) ether
A) alcohol
B) amine
C) aldehyde
D) alkyne
E) ether

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
19
In the 1H NMR spectrum of the following molecule, the protons on which carbon will have the most downfield chemical shift? 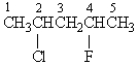
A) 1
B) 2
C) 3
D) 4
E) 5
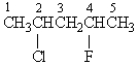
A) 1
B) 2
C) 3
D) 4
E) 5

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
20
The IR spectrum stretching frequency that has the highest energy occurs for which of these bonds?
A) O-H
B) C-O
C) C-Cl
D) C-H
E) C-C
A) O-H
B) C-O
C) C-Cl
D) C-H
E) C-C

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
21
Which molecule will produce two lines in the proton decoupled 13C NMR spectrum?
A) CH3CH2OH
B) CH3OCH3
C) CH3CH2CH2OH
D) CH3OCH2CH3
E)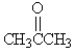
A) CH3CH2OH
B) CH3OCH3
C) CH3CH2CH2OH
D) CH3OCH2CH3
E)
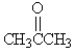

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
22
Which of the following compounds is most likely to absorb ultraviolet radiation in the range of 200 to 400 nm?
A)
B)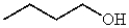
C)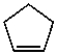
D)
E)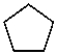
A)

B)
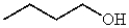
C)

D)

E)


Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
23
Which of the following molecules has more than three (3) different types of protons in its 1H NMR? 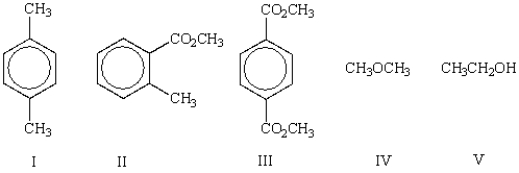
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
24
The compound 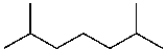 will show what number of peaks in its 1H decoupled 13C NMR spectrum?
will show what number of peaks in its 1H decoupled 13C NMR spectrum?
A) 3
B) 4
C) 5
D) 6
E) 9
 will show what number of peaks in its 1H decoupled 13C NMR spectrum?
will show what number of peaks in its 1H decoupled 13C NMR spectrum?A) 3
B) 4
C) 5
D) 6
E) 9

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
25
A compound with the molecular formula C5H12 gave the following 1H NMR spectrum: singlet, 1.0 (12 H)
The structure of this compound is: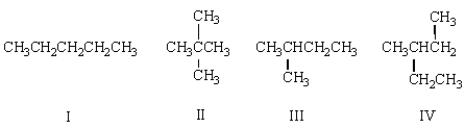
A) I
B) II
C) III
D) IV
E) all are possible
The structure of this compound is:

A) I
B) II
C) III
D) IV
E) all are possible

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
26
Which of the following molecules will show two peaks with an area ratio of 6:4 in its 1H NMR spectrum? 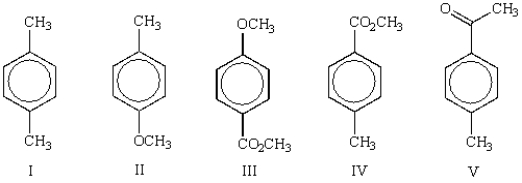
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
27
Which of following will have three peaks in its 1H decoupled 13C NMR spectrum?
A) pentane
B) isopentane
C) isobutane
D) benzene
E) m-chlorotoluene
A) pentane
B) isopentane
C) isobutane
D) benzene
E) m-chlorotoluene

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
28
The following compound will have an 1H NMR spectrum that consists of: 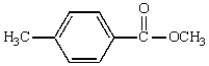
A) two singlets, area ratio 6:4.
B) three singlets, area ratio 3:3:4.
C) four singlets, area ratio 3:3:2:2.
D) two singlets and two doublets, area ratio 3:3:2:2.
E) two triplets and a singlet, area ratio 3:3:4.

A) two singlets, area ratio 6:4.
B) three singlets, area ratio 3:3:4.
C) four singlets, area ratio 3:3:2:2.
D) two singlets and two doublets, area ratio 3:3:2:2.
E) two triplets and a singlet, area ratio 3:3:4.

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
29
The following compound will have a proton NMR spectrum that shows: 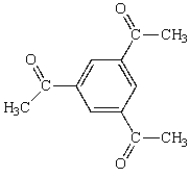
A) two singlets, area ratio 3:1.
B) two singlets, area ratio 9:1.
C) three singlets, area ratio 3:3:6.
D) four singlets, area ratio 3:2:6:1.
E) six singlets, area ratio 3:3:3:1:1:1.

A) two singlets, area ratio 3:1.
B) two singlets, area ratio 9:1.
C) three singlets, area ratio 3:3:6.
D) four singlets, area ratio 3:2:6:1.
E) six singlets, area ratio 3:3:3:1:1:1.

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
30
How many peaks would you expect in the proton decoupled 13C NMR spectrum of 3-bromopentane?
A) 1
B) 2
C) 3
D) 4
E) 5
A) 1
B) 2
C) 3
D) 4
E) 5

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
31
Which of the following molecules would absorb at the longest wavelength in the ultraviolet region?
A) hexane
B) 2-hexene
C) 2,4-hexadiene
D) 1,3,5-hexatriene
E) cyclohexane
A) hexane
B) 2-hexene
C) 2,4-hexadiene
D) 1,3,5-hexatriene
E) cyclohexane

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
32
A compound C3H3Cl5 has a 1H NMR spectrum that consists of a triplet at 4.5 and a doublet at 6.0 (J = 7 Hz) with relative areas 1:2.Its structure is
A) CH3CCl2CCl3
B) CH2ClCCl2CHCl2
C) CHCl2CCl2CCH2Cl
D) CHCl2CHClCHCl2
E) CH2ClCHClCCl3
A) CH3CCl2CCl3
B) CH2ClCCl2CHCl2
C) CHCl2CCl2CCH2Cl
D) CHCl2CHClCHCl2
E) CH2ClCHClCCl3

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
33
A monochloroalkane shows two parent ion peaks m/z at 92 and 94.What is the molecular formula?
A) C4H9Cl
B) C3H7Cl
C) C3H21Cl
D) C4H7Cl
E) C2H6Cl
A) C4H9Cl
B) C3H7Cl
C) C3H21Cl
D) C4H7Cl
E) C2H6Cl

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
34
Compounds that will show only two sharp singlets in their 1H NMR spectra are: 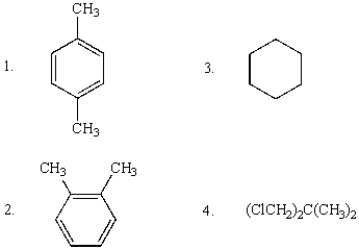
A) 1 and 2
B) 3
C) 1 and 3
D) 1 and 4
E) 1, 2, and 4

A) 1 and 2
B) 3
C) 1 and 3
D) 1 and 4
E) 1, 2, and 4

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
35
The proton(s) on which C will produce a splitting pattern that is a doublet in its 1H NMR spectrum? 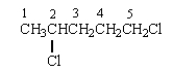
A) 1
B) 2
C) 3
D) 4
E) 5
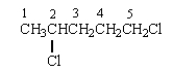
A) 1
B) 2
C) 3
D) 4
E) 5

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
36
The following compounds in order of increasing wavelength of maximum UV-VIS absorption are 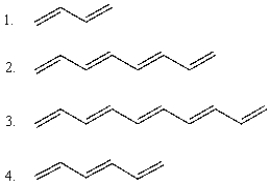
A) 1 < 2 < 3 < 4
B) 1 < 4 < 2 < 3
C) 3 < 2 < 4 < 1
D) 4 < 3 < 2 < 1
E) 1 < 2 < 4 < 3

A) 1 < 2 < 3 < 4
B) 1 < 4 < 2 < 3
C) 3 < 2 < 4 < 1
D) 4 < 3 < 2 < 1
E) 1 < 2 < 4 < 3

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
37
How many peaks would you expect in the proton decoupled 13C NMR spectrum of 2-pentanol?
A) 1
B) 2
C) 3
D) 4
E) 5
A) 1
B) 2
C) 3
D) 4
E) 5

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
38
A bromoalkane shows two parent ion peaks m/z 122 and 124.What is the molecular formula?
A) C4H9Br
B) C3H7Br
C) C2H5Br
D) CH3Br
E) C2H19Br
A) C4H9Br
B) C3H7Br
C) C2H5Br
D) CH3Br
E) C2H19Br

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
39
Which compound has five singlets in its proton-decoupled 13C NMR spectrum and two quartets, one triplet, one doublet, and one singlet in its proton-coupled 13C NMR spectrum?
A)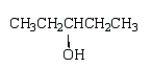
B)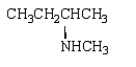
C)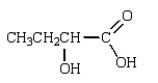
D)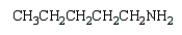
E)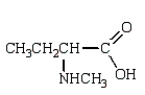
A)

B)
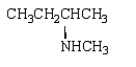
C)

D)
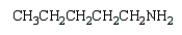
E)


Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
40
Which of the following molecules shows only one singlet in its 1H NMR spectrum? 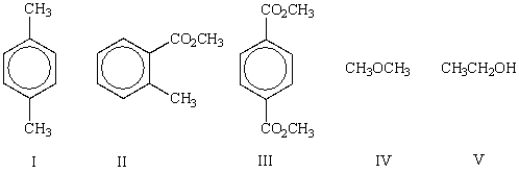
A) I
B) II
C) I and III
D) IV
E) V

A) I
B) II
C) I and III
D) IV
E) V

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
41
Which of the following compounds will have a molecular ion of m/z = 114 and a 13C NMR spectrum with four peaks?
A)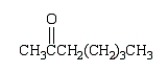
B)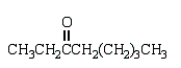
C)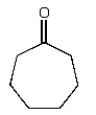
D)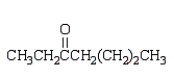
E)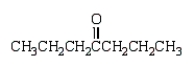
A)
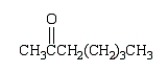
B)
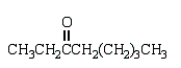
C)

D)
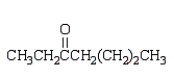
E)
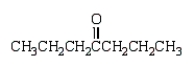

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
42
The mass spectrum of 1-pentanol shows an intense daughter ion peak at m/z = 31.This peak could be due to the formation of:
A) C5H12O•
B) C3H7O+
C) CH3O+
D) CH4O•
E) C4H10O+
A) C5H12O•
B) C3H7O+
C) CH3O+
D) CH4O•
E) C4H10O+

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
43
Which of the following compounds will have one peak in its 1H NMR and two peaks in its 13C NMR spectrum?
A)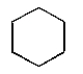
B)
C)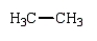
D)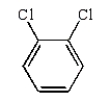
E)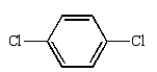
A)

B)

C)
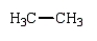
D)

E)


Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
44
An unknown oxygen-containing organic molecule shows a parent peak on its mass spectra at m/z 122.With no information from the visible or uv, the IR revealed major broad absorptions from 2500 cm-1 to 3500 cm-1, and strong sharp absorptions at 2930 cm-1, 1690 cm-1, and 1230 cm-1.The 1H NMR revealed a singlet downfield around 13 (1H) and a multiplet at 7.2 (5H).The molecule consistent with these facts is:
A)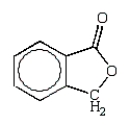
B)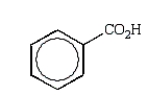
C)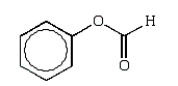
D)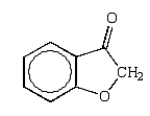
E)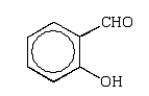
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
45
An unknown oxygen containing organic molecule shows a parent peak in its mass spectrum at m/z 58.There is no measurable uv or visible spectrum.The IR shows strong, sharp absorptions at 2930 cm-1 and 1725 cm-1.The 1H NMR shows a singlet at 2.1 (6H).Which of the following structures best fits the spectral data?
A)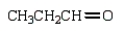
B)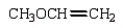
C)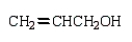
D)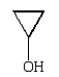
E)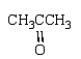
A)
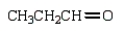
B)
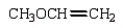
C)
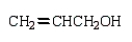
D)

E)
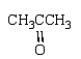

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
46
An unknown hydrocarbon shows a parent peak on its mass spectra at m/z 86.There is no evidence of absorptions from visible or uv spectra.The IR shows no major absorptions outside the C-H stretch and bending vibrations.The 1H NMR reveals a singlet at 0.9 (9H), a triplet at 0.98 (3H), and a quartet at 1.6 (2H).
A)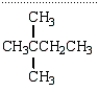
B)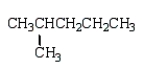
C)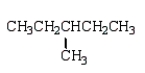
D)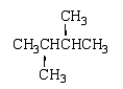
E)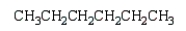
A)
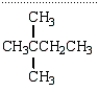
B)
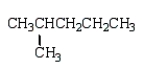
C)

D)

E)
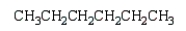

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
47
Which of the following compounds will have two peaks in its 1H NMR spectrum and a broad band in the 3200 to 3500 cm-1 region of its IR spectrum?
A)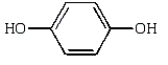
B)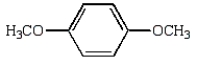
C)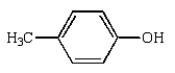
D)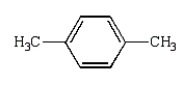
E)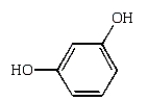
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
48
A hydrocarbon shows a parent ion peak in its mass spectrum at m/z 102.Its 1H NMR spectrum shows only two peaks, a singlet at 2.7 (1H) and a multiplet at 7.4 (5H).The correct structure is:
A)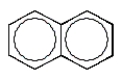
B)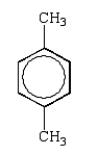
C)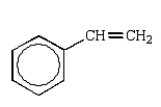
D)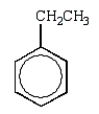
E)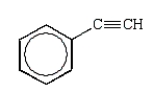
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck



