Deck 5: Stereoisomerism
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/51
Play
Full screen (f)
Deck 5: Stereoisomerism
1
Chiral molecules that have nonsuperimposable mirror images are called:
A) enantiomers
B) diastereomers
C) meso compounds
D) stereogenic
E) symmetrical
A) enantiomers
B) diastereomers
C) meso compounds
D) stereogenic
E) symmetrical
enantiomers
2
How many stereoisomers with the formula CH3CHICHICH3 are possible?
A) 1
B) 2
C) 3
D) 4
E) 5
A) 1
B) 2
C) 3
D) 4
E) 5
3
3
What is the process that separates enantiomers?
A) separation
B) decoupling
C) resetting
D) resolution
E) selective binding
A) separation
B) decoupling
C) resetting
D) resolution
E) selective binding
resolution
4
How many stereogenic centers are present in the following molecule? 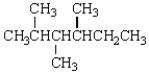
A) 1
B) 2
C) 3
D) 4
E) 5

A) 1
B) 2
C) 3
D) 4
E) 5

Unlock Deck
Unlock for access to all 51 flashcards in this deck.
Unlock Deck
k this deck
5
How many stereoisomers can be obtained from the monobromination of pentane?
A) 1
B) 2
C) 3
D) 4
E) 5
A) 1
B) 2
C) 3
D) 4
E) 5

Unlock Deck
Unlock for access to all 51 flashcards in this deck.
Unlock Deck
k this deck
6
Which of the following molecules has a mirror plane of symmetry? 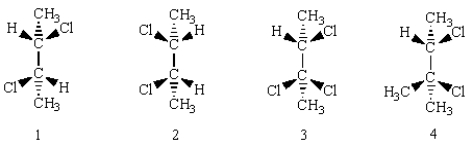
A) 1
B) 2
C) 3
D) 4
E) all of them

A) 1
B) 2
C) 3
D) 4
E) all of them

Unlock Deck
Unlock for access to all 51 flashcards in this deck.
Unlock Deck
k this deck
7
Which of the molecules below has a stereogenic carbon atom? 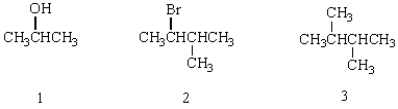
A) 1
B) 2
C) 3
D) 2 and 3
E) 1, 2, and 3

A) 1
B) 2
C) 3
D) 2 and 3
E) 1, 2, and 3

Unlock Deck
Unlock for access to all 51 flashcards in this deck.
Unlock Deck
k this deck
8
Which of the following objects is chiral?
A) egg
B) pencil
C) cross country skis
D) paperclip
E) shoes
A) egg
B) pencil
C) cross country skis
D) paperclip
E) shoes

Unlock Deck
Unlock for access to all 51 flashcards in this deck.
Unlock Deck
k this deck
9
Which of the following statements about enantiomers is INCORRECT?
A) they cannot be differentiated by spectra
B) they have the same melting and boiling points
C) the mirror image of the R enantiomer is the S enantiomer
D) the specific rotation of enantiomers has the same magnitude
E) without exception, S enantiomers will rotate plane-polarized light to the left (counterclockwise)
A) they cannot be differentiated by spectra
B) they have the same melting and boiling points
C) the mirror image of the R enantiomer is the S enantiomer
D) the specific rotation of enantiomers has the same magnitude
E) without exception, S enantiomers will rotate plane-polarized light to the left (counterclockwise)

Unlock Deck
Unlock for access to all 51 flashcards in this deck.
Unlock Deck
k this deck
10
How many stereogenic carbons are in the following molecule? 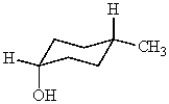
A) 0
B) 1
C) 2
D) 3
E) 4

A) 0
B) 1
C) 2
D) 3
E) 4

Unlock Deck
Unlock for access to all 51 flashcards in this deck.
Unlock Deck
k this deck
11
The total number of possible stereoisomers of 1-bromo-2-chlorocyclopentane is:
A) 2
B) 4
C) 6
D) 8
E) 0
A) 2
B) 4
C) 6
D) 8
E) 0

Unlock Deck
Unlock for access to all 51 flashcards in this deck.
Unlock Deck
k this deck
12
An unknown sample is tested with a polarimeter for optical activity.The results of the test require movement of the analyzer.What samples would give this result?
A) pure enantiomer
B) meso compound
C) racemic mixture
D) both B and C
E) none of these
A) pure enantiomer
B) meso compound
C) racemic mixture
D) both B and C
E) none of these

Unlock Deck
Unlock for access to all 51 flashcards in this deck.
Unlock Deck
k this deck
13
How many chiral stereoisomers can be drawn for CH3CHClCHBrCH3?
A) 1
B) 2
C) 3
D) 4
E) 8
A) 1
B) 2
C) 3
D) 4
E) 8

Unlock Deck
Unlock for access to all 51 flashcards in this deck.
Unlock Deck
k this deck
14
The total number of possible stereoisomers of 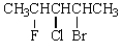 is:
is:
A) 2
B) 4
C) 6
D) 8
E) 0
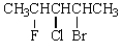 is:
is:A) 2
B) 4
C) 6
D) 8
E) 0

Unlock Deck
Unlock for access to all 51 flashcards in this deck.
Unlock Deck
k this deck
15
How many stereogenic centers are present in the following molecule? 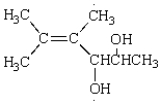
A) 1
B) 2
C) 4
D) 6
E) 8

A) 1
B) 2
C) 4
D) 6
E) 8

Unlock Deck
Unlock for access to all 51 flashcards in this deck.
Unlock Deck
k this deck
16
The number of stereogenic centers in progesterone is: 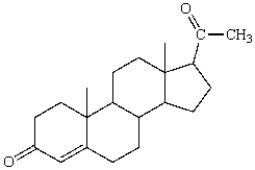 progesterone (no stereochemistry shown)
progesterone (no stereochemistry shown)
A) 2
B) 3
C) 4
D) 5
E) 6
 progesterone (no stereochemistry shown)
progesterone (no stereochemistry shown)A) 2
B) 3
C) 4
D) 5
E) 6

Unlock Deck
Unlock for access to all 51 flashcards in this deck.
Unlock Deck
k this deck
17
An unknown sample is tested with a polarimeter for optical activity.The results of the test required no movement of the analyzer.What samples would give this result?
A) pure enantiomer
B) meso compound
C) racemic mixture
D) both B and C
E) none of these
A) pure enantiomer
B) meso compound
C) racemic mixture
D) both B and C
E) none of these

Unlock Deck
Unlock for access to all 51 flashcards in this deck.
Unlock Deck
k this deck
18
A 50:50 mixture of enantiomers
A) is a meso form.
B) is a pair of diastereomers.
C) is a racemic mixture.
D) rotates plane polarized light.
E) is a pair of conformers.
A) is a meso form.
B) is a pair of diastereomers.
C) is a racemic mixture.
D) rotates plane polarized light.
E) is a pair of conformers.

Unlock Deck
Unlock for access to all 51 flashcards in this deck.
Unlock Deck
k this deck
19
The observed rotation for 100 mL of an aqueous solution containing 1 g of sucrose, placed in a 2-decimeter sample tube, is +1.33° at 25°C.What is the specific rotation of sucrose?
A) +66.5°
B) +266°
C) +41.5
D) +133°
E) 108°
A) +66.5°
B) +266°
C) +41.5
D) +133°
E) 108°

Unlock Deck
Unlock for access to all 51 flashcards in this deck.
Unlock Deck
k this deck
20
The terms that best describe the isomeric relationship between staggered and eclipsed ethane are
A) configurational, achiral, diastereomers.
B) conformational, chiral, enantiomers.
C) conformational, achiral, diastereomers.
D) configurational, chiral, enantiomers.
E) conformational, achiral, enantiomers.
A) configurational, achiral, diastereomers.
B) conformational, chiral, enantiomers.
C) conformational, achiral, diastereomers.
D) configurational, chiral, enantiomers.
E) conformational, achiral, enantiomers.

Unlock Deck
Unlock for access to all 51 flashcards in this deck.
Unlock Deck
k this deck
21
Which of the following statements about the pair of molecules shown below is not true? 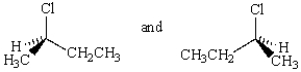
A) They have the same boiling point.
B) One rotates plane polarized light in the opposite direction from the other.
C) They have the same density.
D) One rotates plane polarized light a different number of degrees than the other.
E) They are mirror images of each other.
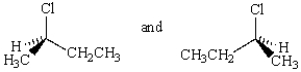
A) They have the same boiling point.
B) One rotates plane polarized light in the opposite direction from the other.
C) They have the same density.
D) One rotates plane polarized light a different number of degrees than the other.
E) They are mirror images of each other.

Unlock Deck
Unlock for access to all 51 flashcards in this deck.
Unlock Deck
k this deck
22
Which of the three molecules below is a diastereomer of the following molecule? 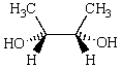
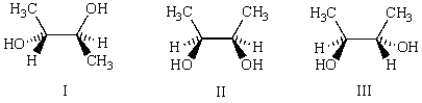
A) I
B) II
C) III
D) there are no diastereomers
E) both I and II


A) I
B) II
C) III
D) there are no diastereomers
E) both I and II

Unlock Deck
Unlock for access to all 51 flashcards in this deck.
Unlock Deck
k this deck
23
According to the R-S convention, which priority is correct for the following sets of groups?
A) NH2 > Cl > CH3 > H
B) Cl > NH2 > CH3 > H
C) Cl > CH3 > NH2 > H
D) H > Cl > CH3 > NH2
E) CH3 > NH2 > Cl > H
A) NH2 > Cl > CH3 > H
B) Cl > NH2 > CH3 > H
C) Cl > CH3 > NH2 > H
D) H > Cl > CH3 > NH2
E) CH3 > NH2 > Cl > H

Unlock Deck
Unlock for access to all 51 flashcards in this deck.
Unlock Deck
k this deck
24
Which of the three molecules below is the enantiomer of the following molecule? 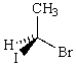
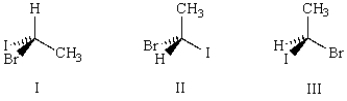
A) I
B) II
C) III
D) there are no enantiomers
E) both II and III


A) I
B) II
C) III
D) there are no enantiomers
E) both II and III

Unlock Deck
Unlock for access to all 51 flashcards in this deck.
Unlock Deck
k this deck
25
The terms that best describe the relationship between (2R,3S)-2-bromo-3-chlorobutane and (2S,3R)-2-bromo-3-chlorobutane are
A) configurational, achiral, diastereomers.
B) conformational, chiral, diastereomers.
C) configurational, chiral, enantiomers.
D) conformational, chiral, enantiomers.
E) configurational, chiral, diastereomers.
A) configurational, achiral, diastereomers.
B) conformational, chiral, diastereomers.
C) configurational, chiral, enantiomers.
D) conformational, chiral, enantiomers.
E) configurational, chiral, diastereomers.

Unlock Deck
Unlock for access to all 51 flashcards in this deck.
Unlock Deck
k this deck
26
The correct IUPAC name for the following molecule is: 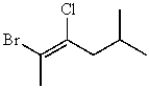
A) (E)-2-bromo-3-chloro-5-methyl-2-hexene
B) (E)-2-bromo-3-chloro-5-methyl-3-hexene
C) (Z)-2-bromo-3-chloro-5-methyl-3-hexene
D) (Z)-2-bromo-3-chloro-5-methyl-2-hexene
E) (E)-5-bromo-4-chloro-2-methyl-4-hexene

A) (E)-2-bromo-3-chloro-5-methyl-2-hexene
B) (E)-2-bromo-3-chloro-5-methyl-3-hexene
C) (Z)-2-bromo-3-chloro-5-methyl-3-hexene
D) (Z)-2-bromo-3-chloro-5-methyl-2-hexene
E) (E)-5-bromo-4-chloro-2-methyl-4-hexene

Unlock Deck
Unlock for access to all 51 flashcards in this deck.
Unlock Deck
k this deck
27
Which name describes the following structure? 
A) (R)-3-methyl-1-penten-3-ol
B) (S)-3-methyl-1-penten-3-ol
C) (R)-3-ethyl-1-buten-3-ol
D) (R)-3-methyl-1-pentyn-3-ol
E) (S)-3-ethyl-1-buten-3-ol

A) (R)-3-methyl-1-penten-3-ol
B) (S)-3-methyl-1-penten-3-ol
C) (R)-3-ethyl-1-buten-3-ol
D) (R)-3-methyl-1-pentyn-3-ol
E) (S)-3-ethyl-1-buten-3-ol

Unlock Deck
Unlock for access to all 51 flashcards in this deck.
Unlock Deck
k this deck
28
Which of the following would constitute a pair of enantiomers?
A) staggered and eclipsed forms of ethane
B) cis and trans-2-butene
C) meso- and (2R,3R)-2,3-dibromobutane
D) (2R,3R) and (2S,3S)-tartaric acid
E) none of these
A) staggered and eclipsed forms of ethane
B) cis and trans-2-butene
C) meso- and (2R,3R)-2,3-dibromobutane
D) (2R,3R) and (2S,3S)-tartaric acid
E) none of these

Unlock Deck
Unlock for access to all 51 flashcards in this deck.
Unlock Deck
k this deck
29
(R)-2-chlorobutane is correctly represented by which of the following: 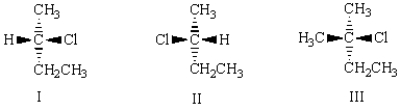
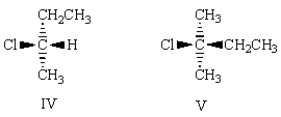
A) I
B) II
C) III
D) IV
E) V


A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 51 flashcards in this deck.
Unlock Deck
k this deck
30
The priority order for R/S nomenclature is
A) -CH=CH2 > -OH > -CH3 > -CH2CH3
B) -OH > -CH2CH3 > -CH=CH2 > -CH3
C) -OH > -CH=CH2 > -CH2CH3 > -CH3
D) -CH3 > -CH2CH3 > -CH=CH2 > -OH
E) -CH2CH3 > -CH3 > -CH=CH2 > -OH
A) -CH=CH2 > -OH > -CH3 > -CH2CH3
B) -OH > -CH2CH3 > -CH=CH2 > -CH3
C) -OH > -CH=CH2 > -CH2CH3 > -CH3
D) -CH3 > -CH2CH3 > -CH=CH2 > -OH
E) -CH2CH3 > -CH3 > -CH=CH2 > -OH

Unlock Deck
Unlock for access to all 51 flashcards in this deck.
Unlock Deck
k this deck
31
Which of the following groups has the highest priority for assigning R-S absolute configuration?
A) CH2=CH-
B) (CH3)2CH-
C) (CH3)3C-
D) CH3CH2-
E) CH3-
A) CH2=CH-
B) (CH3)2CH-
C) (CH3)3C-
D) CH3CH2-
E) CH3-

Unlock Deck
Unlock for access to all 51 flashcards in this deck.
Unlock Deck
k this deck
32
What is correct name for the following structure? 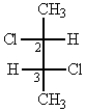
A) (R,S)-2,3-dichlorobutane
B) (2R,3S)-2,3-dichlorobutane
C) (2S,3S)-2,3-dichlorobutane
D) (2R,3R)-2,3-dichlorobutane
E) none of these

A) (R,S)-2,3-dichlorobutane
B) (2R,3S)-2,3-dichlorobutane
C) (2S,3S)-2,3-dichlorobutane
D) (2R,3R)-2,3-dichlorobutane
E) none of these

Unlock Deck
Unlock for access to all 51 flashcards in this deck.
Unlock Deck
k this deck
33
Of the following structures, how many are classified "E"? 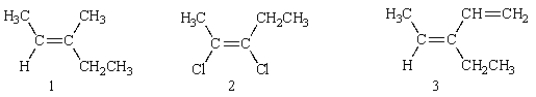
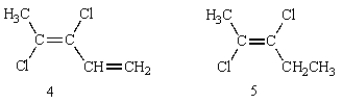
A) 1
B) 2
C) 3
D) 4
E) 5

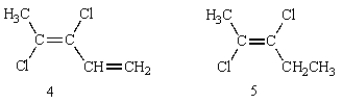
A) 1
B) 2
C) 3
D) 4
E) 5

Unlock Deck
Unlock for access to all 51 flashcards in this deck.
Unlock Deck
k this deck
34
Of the following structures, how many are classified "Z"? 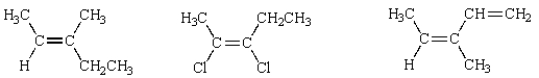
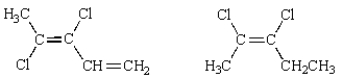
A) 1
B) 2
C) 3
D) 4
E) 5

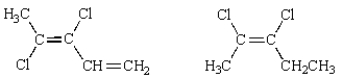
A) 1
B) 2
C) 3
D) 4
E) 5

Unlock Deck
Unlock for access to all 51 flashcards in this deck.
Unlock Deck
k this deck
35
Which of the following structures depicts (R)-3-ethyl-2,3-dimethylhexane?
A)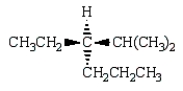
B)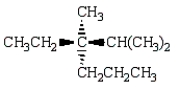
C)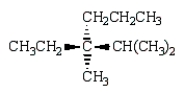
D)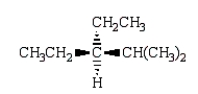
E)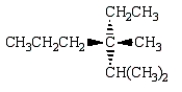
A)
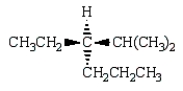
B)

C)

D)

E)


Unlock Deck
Unlock for access to all 51 flashcards in this deck.
Unlock Deck
k this deck
36
Which of the following molecules are the same? 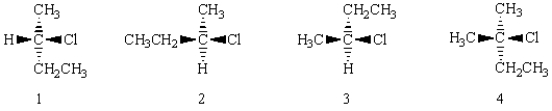
A) 1 and 2
B) 3 and 4
C) 1 and 3
D) 2 and 3
E) 2 and 4
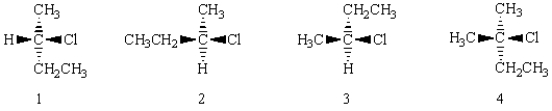
A) 1 and 2
B) 3 and 4
C) 1 and 3
D) 2 and 3
E) 2 and 4

Unlock Deck
Unlock for access to all 51 flashcards in this deck.
Unlock Deck
k this deck
37
Which of the following are achiral conformers?
A) staggered and eclipsed forms of ethane
B) cis and trans-2-butene
C) meso and (2R,3R)-2,3-dibromobutane
D) (R) and (S)-lactic acid
A) staggered and eclipsed forms of ethane
B) cis and trans-2-butene
C) meso and (2R,3R)-2,3-dibromobutane
D) (R) and (S)-lactic acid

Unlock Deck
Unlock for access to all 51 flashcards in this deck.
Unlock Deck
k this deck
38
Which name describes the following structure? 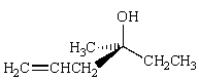
A) (R)-4-methyl-1-hexen-4-ol
B) (S)-4-ethyl-1-penten-4-ol
C) (R)-4-ethyl-1-penten-3-ol
D) (S)-4-methyl-1-hexen-4-ol
E) (S)-4-methyl-1-hexyn-4-ol

A) (R)-4-methyl-1-hexen-4-ol
B) (S)-4-ethyl-1-penten-4-ol
C) (R)-4-ethyl-1-penten-3-ol
D) (S)-4-methyl-1-hexen-4-ol
E) (S)-4-methyl-1-hexyn-4-ol

Unlock Deck
Unlock for access to all 51 flashcards in this deck.
Unlock Deck
k this deck
39
The terms that best describe the relationship between (2R,3S)-2,3-butanediol and (2S,3S)-2,3-butanediol are
A) configurational, diastereomers.
B) conformational, enantiomers.
C) conformational, diastereomers
D) configurational, enantiomers.
E) configurational, cis/trans.
A) configurational, diastereomers.
B) conformational, enantiomers.
C) conformational, diastereomers
D) configurational, enantiomers.
E) configurational, cis/trans.

Unlock Deck
Unlock for access to all 51 flashcards in this deck.
Unlock Deck
k this deck
40
Which of the following structures is (E)-2,3-dichloro-2-pentene? 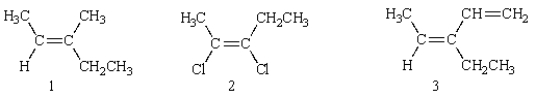
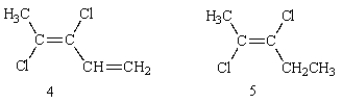
A) 1
B) 2
C) 3
D) 4
E) 5

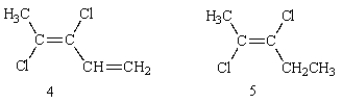
A) 1
B) 2
C) 3
D) 4
E) 5

Unlock Deck
Unlock for access to all 51 flashcards in this deck.
Unlock Deck
k this deck
41
When (R)-3-bromo-2-methyl-1-butene is reacted with HBr, two stereoisomers are formed.What is the relationship of these stereoisomers?
A) enantiomers
B) meso compounds
C) diastereomers
D) E
E) Z
A) enantiomers
B) meso compounds
C) diastereomers
D) E
E) Z

Unlock Deck
Unlock for access to all 51 flashcards in this deck.
Unlock Deck
k this deck
42
When (S)-3-bromo-1-butene is treated with HBr, two stereoisomeric products form.What is the relationship of these two products?
A) enantiomers
B) diastereomers
C) meso compounds
D) racemic mixture
E) cis/trans
A) enantiomers
B) diastereomers
C) meso compounds
D) racemic mixture
E) cis/trans

Unlock Deck
Unlock for access to all 51 flashcards in this deck.
Unlock Deck
k this deck
43
Which one of the following structures represents a meso compound?
A)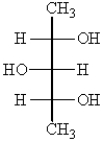
B)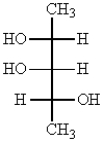
C)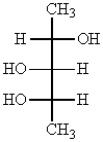
D)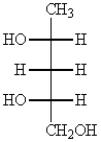
E)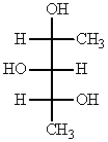
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 51 flashcards in this deck.
Unlock Deck
k this deck
44
The Fischer projection that represents the same molecule as 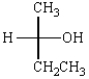 is:
is:
A)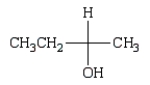
B)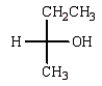
C)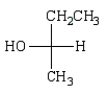
D)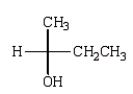
E)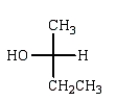
 is:
is:A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 51 flashcards in this deck.
Unlock Deck
k this deck
45
Treating 1-butene with HBr produces a product with one stereogenic carbon.What is the name of the product?
A) 2-bromo-1-butene
B) 1-bromobutane
C) (R)-2-bromobutane
D) (S)-2-bromobutane
E) both C and D in equal amounts
A) 2-bromo-1-butene
B) 1-bromobutane
C) (R)-2-bromobutane
D) (S)-2-bromobutane
E) both C and D in equal amounts

Unlock Deck
Unlock for access to all 51 flashcards in this deck.
Unlock Deck
k this deck
46
How many stereogenic carbons are produced from the following sequence of reactions? 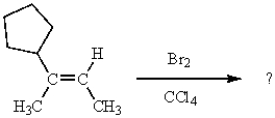
A) 1
B) 2
C) 3
D) 4
E) 5

A) 1
B) 2
C) 3
D) 4
E) 5

Unlock Deck
Unlock for access to all 51 flashcards in this deck.
Unlock Deck
k this deck
47
The absolute configuration around the stereogenic center of the molecule below is: 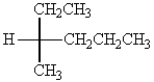
A) R
B) S
C) E
D) Z
E) trans

A) R
B) S
C) E
D) Z
E) trans

Unlock Deck
Unlock for access to all 51 flashcards in this deck.
Unlock Deck
k this deck
48
Enantiomers may differ in the following property:
A) the rate at which they react with a chiral reagent
B) boiling point
C) melting point
D) number of degrees they rotate plane polarized light
E) solubility in water
A) the rate at which they react with a chiral reagent
B) boiling point
C) melting point
D) number of degrees they rotate plane polarized light
E) solubility in water

Unlock Deck
Unlock for access to all 51 flashcards in this deck.
Unlock Deck
k this deck
49
The product of addition of bromine to (R)-3-buten-2-ol will be
A) a 50:50 mixture of enantiomers.
B) a mixture of enantiomers formed in unequal amounts.
C) a 50:50 mixture of diastereomers.
D) a mixture of diastereomers formed in unequal amounts.
E) optically inactive.
A) a 50:50 mixture of enantiomers.
B) a mixture of enantiomers formed in unequal amounts.
C) a 50:50 mixture of diastereomers.
D) a mixture of diastereomers formed in unequal amounts.
E) optically inactive.

Unlock Deck
Unlock for access to all 51 flashcards in this deck.
Unlock Deck
k this deck
50
What is the absolute configuration around C-2 and C-3? 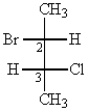
A) R, R
B) S, S
C) R, S
D) S, R
E) E, Z

A) R, R
B) S, S
C) R, S
D) S, R
E) E, Z

Unlock Deck
Unlock for access to all 51 flashcards in this deck.
Unlock Deck
k this deck
51
The Fischer projection formula for (S)-lactic acid (2-hydroxypropanoic acid) is
A)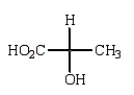
B)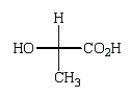
C)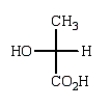
D)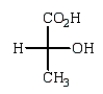
E)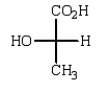
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 51 flashcards in this deck.
Unlock Deck
k this deck



