Deck 3: Alkenes and Alkynes
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/52
Play
Full screen (f)
Deck 3: Alkenes and Alkynes
1
Which of the following molecular formulas could not represent an alkene?
A) C5H10
B) C7H14
C) C10H20
D) C27H56
E) C31H62
A) C5H10
B) C7H14
C) C10H20
D) C27H56
E) C31H62
C27H56
2
Which of the following molecules is 4-methyl-2-hexyne?
A)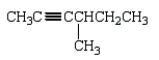
B)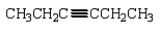
C)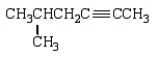
D)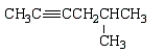
E)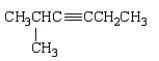
A)

B)
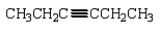
C)
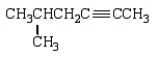
D)
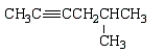
E)
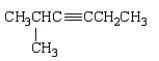

3
The multiple bonds in the following compounds are conjugated: 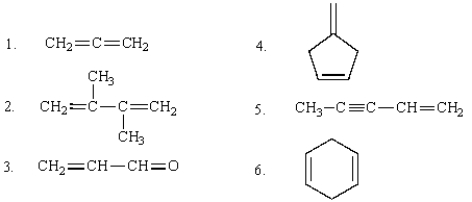
A) 2, 3, and 5
B) 4 and 6
C) only 1
D) 2 and 3
E) 2 and 5

A) 2, 3, and 5
B) 4 and 6
C) only 1
D) 2 and 3
E) 2 and 5
2, 3, and 5
4
What is the percent s character in an sp hybrid orbital?
A) 25%
B) 33%
C) 50%
D) 67%
E) 75%
A) 25%
B) 33%
C) 50%
D) 67%
E) 75%

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
5
Which of the following dienes can be classified as conjugated?
A)CH3CH=C=CH2
B)CH3CH=CHCH=CH2
C)CH2=CHCH2CH=CH2
D)CH3CH=CHCH2CH2CH=CH2
E)CH2=C=CH2
A)CH3CH=C=CH2
B)CH3CH=CHCH=CH2
C)CH2=CHCH2CH=CH2
D)CH3CH=CHCH2CH2CH=CH2
E)CH2=C=CH2

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
6
Which of the following hydrocarbons will be the most acidic?
A) pentane
B) ethene
C) acetylene
D) isobutane
E) propylene
A) pentane
B) ethene
C) acetylene
D) isobutane
E) propylene

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
7
What is the correct structure for 2,3-dimethyl-2-pentene?
A)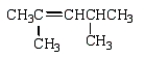
B)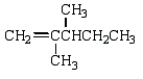
C)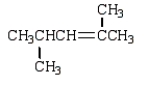
D)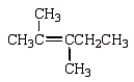
E)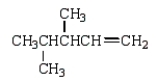
A)
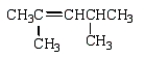
B)

C)

D)

E)


Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
8
Which of the following statements are true about acetylene (ethyne)?
A) It is more acidic than ethane and ethene.
B) The bond angle around the carbon-carbon triple bond is 180°.
C) The carbon-carbon triple bond is shorter than the carbon-carbon double bond.
D) All of the above are true.
E) None of the above are true.
A) It is more acidic than ethane and ethene.
B) The bond angle around the carbon-carbon triple bond is 180°.
C) The carbon-carbon triple bond is shorter than the carbon-carbon double bond.
D) All of the above are true.
E) None of the above are true.

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
9
The correct IUPAC name for the following molecule is: 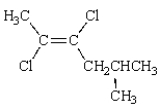
A) trans-2,3-dichloro-5-methyl-2-hexene
B) trans-2,3-dichloro-5-methyl-3-hexene
C) cis-2,3-dichloro-5-methyl-3-hexene
D) trans-4,5-dichloro-2-methyl-4-hexene
E) cis-4,5-dichloro-2-methyl-4-hexene

A) trans-2,3-dichloro-5-methyl-2-hexene
B) trans-2,3-dichloro-5-methyl-3-hexene
C) cis-2,3-dichloro-5-methyl-3-hexene
D) trans-4,5-dichloro-2-methyl-4-hexene
E) cis-4,5-dichloro-2-methyl-4-hexene

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
10
What is the percent s character in an sp2 hybrid orbital?
A) 25%
B) 33%
C) 50%
D) 67%
E) 75%
A) 25%
B) 33%
C) 50%
D) 67%
E) 75%

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
11
Which of the following statements is false relative to alkenes?
A) the C of the carbon-carbon double bond is sp2 hybridized
B) the bond angles are approximately 120° around the carbon-carbon double bond
C) there is the possibility of cis/trans isomerism
D) they are more reactive than alkanes
E) the bond length of the carbon-carbon double bond is longer than that of the carbon-carbon single bond
A) the C of the carbon-carbon double bond is sp2 hybridized
B) the bond angles are approximately 120° around the carbon-carbon double bond
C) there is the possibility of cis/trans isomerism
D) they are more reactive than alkanes
E) the bond length of the carbon-carbon double bond is longer than that of the carbon-carbon single bond

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
12
The correct name of the molecule below is: 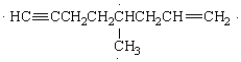
A) 5-methyl-7-octen-1-yne
B) 4-methyl-1-octen-7-yne
C) 4-methyl-1-octyn-7-ene
D) 5-methyl-1-octen-7-yne
E) none of these is correct
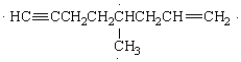
A) 5-methyl-7-octen-1-yne
B) 4-methyl-1-octen-7-yne
C) 4-methyl-1-octyn-7-ene
D) 5-methyl-1-octen-7-yne
E) none of these is correct

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
13
Which of the following compounds can exhibit cis/trans isomerism?
A) 1-heptene
B) 2-heptene
C) 2-methyl-2-hexene
D) 3-methyl-1-hexene
E) 3-ethyl-2-pentene
A) 1-heptene
B) 2-heptene
C) 2-methyl-2-hexene
D) 3-methyl-1-hexene
E) 3-ethyl-2-pentene

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
14
Which compound contains a carbon-carbon double bond with (Z) stereochemistry?
A)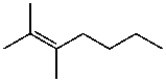
B)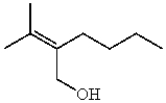
C)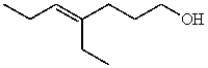
D)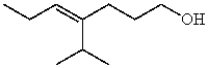
E)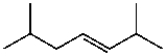
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
15
The double bond in ethene is made up of
A) a pi bond and a sigma bond formed by lateral overlap of two p orbitals.
B) a sigma bond formed by overlap of two s orbitals and a pi bond formed by lateral overlap of two p orbitals.
C) a pi bond formed by end-on overlap of two sp2 orbitals and a sigma bond formed by overlap of two s orbitals.
D) a sigma bond formed by end-on overlap of two sp2 orbitals and a pi bond formed by lateral overlap of two p orbitals.
E) a pi bond formed by lateral overlap of two sp2 orbitals and a sigma bond formed by end-on overlap of two sp2 orbitals.
A) a pi bond and a sigma bond formed by lateral overlap of two p orbitals.
B) a sigma bond formed by overlap of two s orbitals and a pi bond formed by lateral overlap of two p orbitals.
C) a pi bond formed by end-on overlap of two sp2 orbitals and a sigma bond formed by overlap of two s orbitals.
D) a sigma bond formed by end-on overlap of two sp2 orbitals and a pi bond formed by lateral overlap of two p orbitals.
E) a pi bond formed by lateral overlap of two sp2 orbitals and a sigma bond formed by end-on overlap of two sp2 orbitals.

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
16
The structure of (Z)-3-methyl-2-pentene is
A)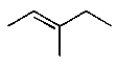
B)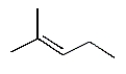
C)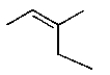
D)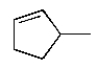
E)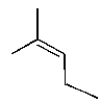
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
17
What is the correct name for the following molecule? 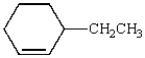
A) 1-ethylcyclohexene
B) 2-ethylcyclohexene
C) 3-ethylcyclohexene
D) cyclohexylethane
E) 1-ethyl-3-cyclohexene

A) 1-ethylcyclohexene
B) 2-ethylcyclohexene
C) 3-ethylcyclohexene
D) cyclohexylethane
E) 1-ethyl-3-cyclohexene

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
18
The triple bond in ethyne is made up of
A) two pi bonds and a sigma bond, each formed by a lateral overlap of two p orbitals.
B) a sigma bond formed by overlap of two s orbitals and two pi bonds, each formed by lateral overlap of two p orbitals.
C) a sigma bond formed by end-on overlap of two sp2 orbitals and a pi bond formed by lateral overlap of two p orbitals.
D) two pi bonds, each formed by lateral overlap of two p orbitals, and a sigma bond formed by end-on overlap of two sp orbitals.
E) two pi bonds, each formed by end-on overlap of two p orbitals, and a sigma bond formed by lateral overlap of two sp orbitals.
A) two pi bonds and a sigma bond, each formed by a lateral overlap of two p orbitals.
B) a sigma bond formed by overlap of two s orbitals and two pi bonds, each formed by lateral overlap of two p orbitals.
C) a sigma bond formed by end-on overlap of two sp2 orbitals and a pi bond formed by lateral overlap of two p orbitals.
D) two pi bonds, each formed by lateral overlap of two p orbitals, and a sigma bond formed by end-on overlap of two sp orbitals.
E) two pi bonds, each formed by end-on overlap of two p orbitals, and a sigma bond formed by lateral overlap of two sp orbitals.

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
19
The correct name for 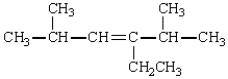 is:
is:
A) 2-methyl-4-isopropyl-3-hexene.
B) 3-ethyl-2,5-dimethyl-3-hexene.
C) 2,5-dimethyl-4-ethyl-3-hexene.
D) 1-ethyl-1,2-diisopropylethene.
E) 1,2-diisopropyl-1-butene.
 is:
is:A) 2-methyl-4-isopropyl-3-hexene.
B) 3-ethyl-2,5-dimethyl-3-hexene.
C) 2,5-dimethyl-4-ethyl-3-hexene.
D) 1-ethyl-1,2-diisopropylethene.
E) 1,2-diisopropyl-1-butene.

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
20
The correct structure for allyl bromide is:
A)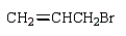
B)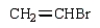
C)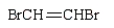
D)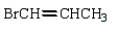
E)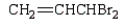
A)
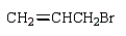
B)
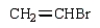
C)
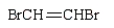
D)
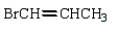
E)
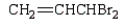

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
21
Select the necessary reagents to convert 1-methylcyclopentene to 1-methylcyclopentanol. 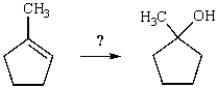
A) H2O and H2SO4
B) Zn, H2O
C) BH3, then H2O2 and -OH
D) O3, then Zn, H+
E) KOH in alcohol and heat
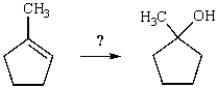
A) H2O and H2SO4
B) Zn, H2O
C) BH3, then H2O2 and -OH
D) O3, then Zn, H+
E) KOH in alcohol and heat

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
22
Addition of H2 to 2-pentyne in the presence of the Lindlar's catalyst will produce:
A) pentane
B) 1-pentene
C) cis-2-pentene
D) trans-2-pentene
A) pentane
B) 1-pentene
C) cis-2-pentene
D) trans-2-pentene

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
23
The products of the following reaction sequence 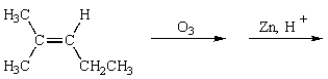 are
are
A)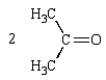
B)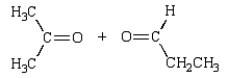
C)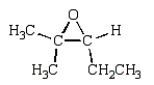
D)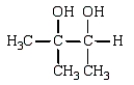
E)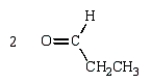
 are
areA)

B)
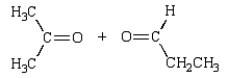
C)

D)

E)


Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
24
The product of the reaction  is:
is:
A)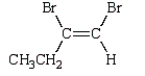
B)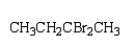
C)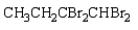
D)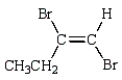
E)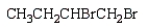
 is:
is:A)

B)
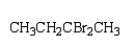
C)
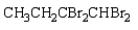
D)

E)
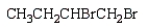

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
25
What is the product for the reaction below? 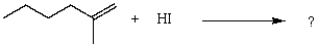
A)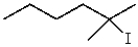
B)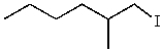
C)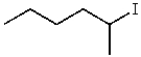
D)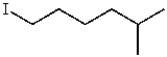
E)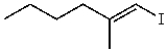
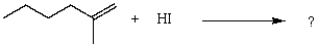
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
26
The products obtained by the acid-catalyzed hydration of 1-methylcyclohexene and methylenecyclohexene are
A) identical.
B) regioisomers.
C) cis-trans isomers.
D) constitutional isomers.
E) none of the above.
A) identical.
B) regioisomers.
C) cis-trans isomers.
D) constitutional isomers.
E) none of the above.

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
27
The Diels-Alder reaction is very important in the synthesis of six-membered rings.What six-membered ring is produced with the following reaction? 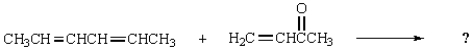
A)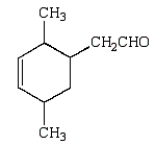
B)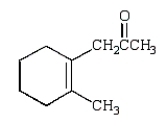
C)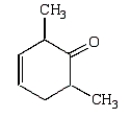
D)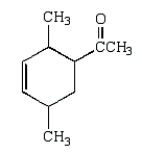
E)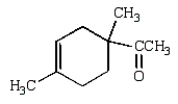
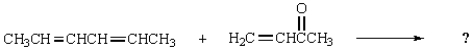
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
28
What is observed when water, in the presence of sulfuric acid, is added to 2-methyl-2-pentene?
A)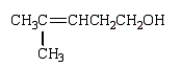
B)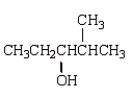
C)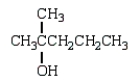
D)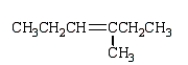
E)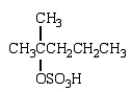
A)
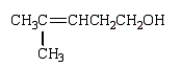
B)

C)
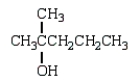
D)

E)


Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
29
Select the necessary reagent(s) to convert cycloheptene to cycloheptane. 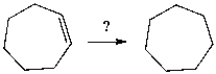
A) H2 and Ni
B) H2O
C) H2SO4 and heat
D) Zn and H+
E) KOH in alcohol and heat
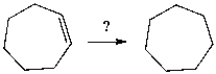
A) H2 and Ni
B) H2O
C) H2SO4 and heat
D) Zn and H+
E) KOH in alcohol and heat

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
30
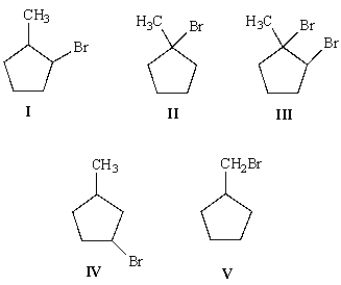
Upon ozonolysis and treatment with Zn in water, compound A yielded two moles of formaldehyde, HCHO, and 1 mole of the following molecule: 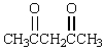 What is the structure of A?
What is the structure of A? 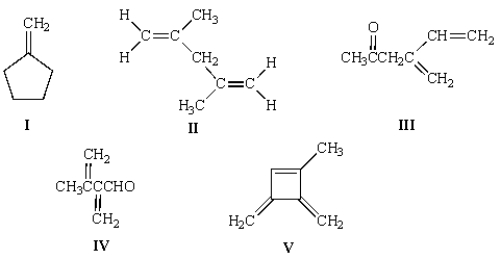
A) I
B) II
C) III
D) IV
E) V
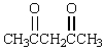 What is the structure of A?
What is the structure of A? 
A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
31
The product of the reaction 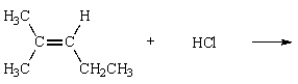 is
is
A)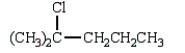
B)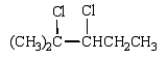
C)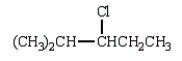
D)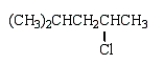
E)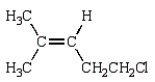
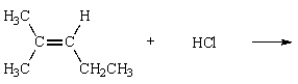 is
isA)
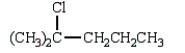
B)
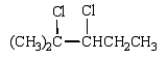
C)

D)
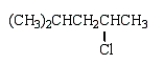
E)


Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
32
What product(s) will be observed by the addition of one molar equivalent of Br2 to ________________ 1,3-cyclohexadiene?
A) 1,2-dibromocyclohexene
B) 3,4-dibromocyclohexene
C) 1,3-dibromocyclohexene
D) 3,6-dibromocyclohexene
E) both b and d
A) 1,2-dibromocyclohexene
B) 3,4-dibromocyclohexene
C) 1,3-dibromocyclohexene
D) 3,6-dibromocyclohexene
E) both b and d

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
33
The product of the reaction sequence 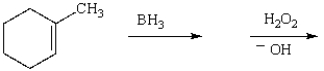 is
is
A)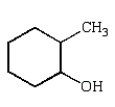
B)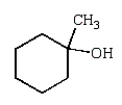
C)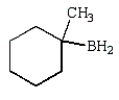
D)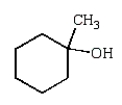
E) none of the above
 is
isA)

B)

C)

D)

E) none of the above

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
34
What would be the major product of the following reaction? 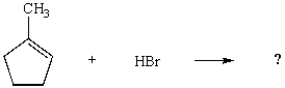
A) I
B) II
C) III
D) IV
E) V


A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
35
What is the name of the product formed from the following reaction? 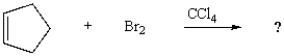
A) bromocyclopentane
B) 1,1-dibromocyclopentane
C) cis-1,2-dibromocyclopentane
D) trans-1,2-dibromocyclopentane
E) 1,1-dibromocyclopentene
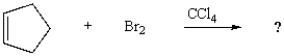
A) bromocyclopentane
B) 1,1-dibromocyclopentane
C) cis-1,2-dibromocyclopentane
D) trans-1,2-dibromocyclopentane
E) 1,1-dibromocyclopentene

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
36
What is the product for the reaction below? 
A)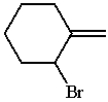
B)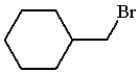
C)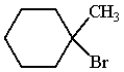
D)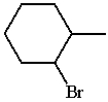
E) none of these

A)

B)

C)

D)

E) none of these

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
37
The products obtained by adding 1 mole of HBr to 2,4-hexadiene are
A) 4-bromo-2-hexene and 5-bromo-2-hexene.
B) 3-bromo-2-hexene and 4-bromo-2-hexene.
C) 4-bromo-2-hexene and 2-bromo-4-hexene.
D) 2-bromo-3-hexene and 3-bromo-2-hexene.
E) 2-bromo-3-hexene and 4-bromo-2-hexene.
A) 4-bromo-2-hexene and 5-bromo-2-hexene.
B) 3-bromo-2-hexene and 4-bromo-2-hexene.
C) 4-bromo-2-hexene and 2-bromo-4-hexene.
D) 2-bromo-3-hexene and 3-bromo-2-hexene.
E) 2-bromo-3-hexene and 4-bromo-2-hexene.

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
38
The product of the reaction 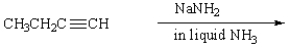 is:
is:
A)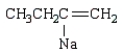
B)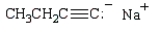
C)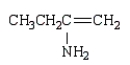
D)
E)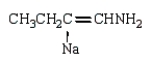
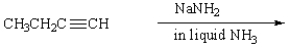 is:
is:A)
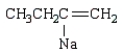
B)
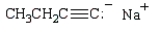
C)
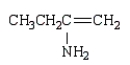
D)

E)
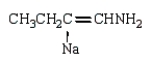

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
39
The product of addition of two moles of HBr to 1,4-pentadiene is
A) 2,2-dibromopentane.
B) 2,4-dibromopentane.
C) 1,5-dibromopentane.
D) 3,3-dibromopentane.
E) 1,4-dibromopentane.
A) 2,2-dibromopentane.
B) 2,4-dibromopentane.
C) 1,5-dibromopentane.
D) 3,3-dibromopentane.
E) 1,4-dibromopentane.

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
40
What type of compound is prepared by adding water to propyne in the presence of sulfuric acid and mercuric sulfate?
A) aldehyde
B) ketone
C) carboxylic acid
D) ester
E) ether
A) aldehyde
B) ketone
C) carboxylic acid
D) ester
E) ether

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
41
Upon ozonolysis which alkene will give only acetone, (CH3)2C=O?
A) 2,3-dimethyl-2-butene
B) 2,2-dimethyl-2-butene
C) 3-hexene
D) 2-methyl-2-pentene
E) 2-methyl-3-hexene
A) 2,3-dimethyl-2-butene
B) 2,2-dimethyl-2-butene
C) 3-hexene
D) 2-methyl-2-pentene
E) 2-methyl-3-hexene

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
42
What is/are the final product(s) in the following multistep synthesis?
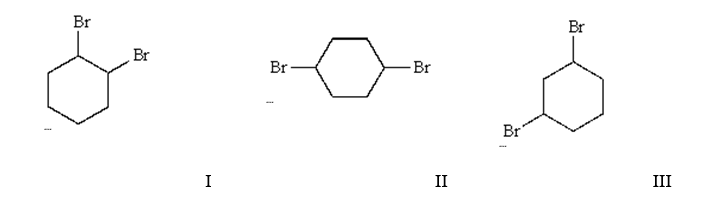
A) I
B) II
C) III
D) I and II
E) all are produced

A) I
B) II
C) III
D) I and II
E) all are produced

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
43
What is the name of the alkene produced by treating 1-butyne with 1 mole of Br2?
A) (E)-1,2-dibromo-1-butene
B) 1,1-dibromo-1-butene
C) (Z)-1,2-dibromo-1-butene
D) 1,2-dibromo-1-butene
E) none of the above
A) (E)-1,2-dibromo-1-butene
B) 1,1-dibromo-1-butene
C) (Z)-1,2-dibromo-1-butene
D) 1,2-dibromo-1-butene
E) none of the above

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
44
What alkene is required to make 3-methyl-1-butanol using the hydroboration-oxidation reaction?
A) 1-butene
B) 2-butene
C) 3-methyl-1-butene
D) 2-methyl-2-butene
E) 2-methyl-1-butene
A) 1-butene
B) 2-butene
C) 3-methyl-1-butene
D) 2-methyl-2-butene
E) 2-methyl-1-butene

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
45
Which of the following alkenes is needed to prepare 3-cyclohexyl-1-propanol via a hydroboration-oxidation reaction? 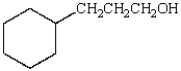 3-cyclohexyl-1-propanol
3-cyclohexyl-1-propanol
A) cyclohexene
B) vinyl cyclohexane
C) allyl cyclohexane
D) propyl cyclohexene
E) 1- octene
 3-cyclohexyl-1-propanol
3-cyclohexyl-1-propanolA) cyclohexene
B) vinyl cyclohexane
C) allyl cyclohexane
D) propyl cyclohexene
E) 1- octene

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
46
What is the final product of adding 1 mole of each reactant in the following sequence? 
A) propyl chloride
B) propyl bromide
C) 1-bromo-2-chloropropane
D) 2-bromo-2-chloropropane
E) 2,2-dibromopropane

A) propyl chloride
B) propyl bromide
C) 1-bromo-2-chloropropane
D) 2-bromo-2-chloropropane
E) 2,2-dibromopropane

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
47
What type of carbocation will form from the addition of a H+ to 2-methylpropene?
A) H3C+
B) 1°
C) 2°
D) 3°
E) allyl
A) H3C+
B) 1°
C) 2°
D) 3°
E) allyl

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
48
Polyethylene is usually produced by
A) an ionic electrophilic addition reaction
B) heating ethylene to 1000oC.
C) cationic polymerization.
D) a free-radical chain reaction.
E) epoxidation.
A) an ionic electrophilic addition reaction
B) heating ethylene to 1000oC.
C) cationic polymerization.
D) a free-radical chain reaction.
E) epoxidation.

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
49
The first step in the free radical mechanism for the preparation of polyethylene is:
A) formation of a stable carbocation
B) formation of a stable carbanion
C) heating an organic peroxide to break the O-O bond
D) decoupling of the free radicals
E) propagation of the free radicals
A) formation of a stable carbocation
B) formation of a stable carbanion
C) heating an organic peroxide to break the O-O bond
D) decoupling of the free radicals
E) propagation of the free radicals

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
50
Which of the following reagents can be used to distinguish cyclohexene from cyclohexane?
A) Zn, H+
B) H2O
C) Cl2, h
D) Br2, CCl4
E) O2, heat
A) Zn, H+
B) H2O
C) Cl2, h
D) Br2, CCl4
E) O2, heat

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
51
Markovnikov addition of HCl to propene involves:
A) initial attack by the chloride ion
B) initial attack by the chlorine atom
C) isomerization of 1-chloropropane
D) formation of a propyl cation
E) formation of an isopropyl cation
A) initial attack by the chloride ion
B) initial attack by the chlorine atom
C) isomerization of 1-chloropropane
D) formation of a propyl cation
E) formation of an isopropyl cation

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
52
Cyclohexene is treated with cold dilute KMnO4.What is the spatial arrangement of the hydroxyls on the resulting cyclohexane ring?
A) 1,2-cis
B) 1,2-trans
C) 1,4-cis
D) 1,4-trans
E) a mixture of 1,2-cis and 1,2-trans
A) 1,2-cis
B) 1,2-trans
C) 1,4-cis
D) 1,4-trans
E) a mixture of 1,2-cis and 1,2-trans

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck



