Deck 5: Light
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/100
Play
Full screen (f)
Deck 5: Light
1
What wavelengths of light can the human eye see?
A) 3.8-7.5 m
B) 3.8-7.5 nm
C) 380-750 cm
D) 380-750 nm
E) 3.8-7.5 m
A) 3.8-7.5 m
B) 3.8-7.5 nm
C) 380-750 cm
D) 380-750 nm
E) 3.8-7.5 m
380-750 nm
2
The fact that the speed of light is constant as it travels through a vacuum means that
A) photons with longer wavelengths have lower frequencies.
B) radio wave photons have shorter wavelengths than gamma-ray photons.
C) X-rays can be transmitted through the atmosphere around the world.
D) ultraviolet photons have less energy than visible photons.
E) all photons have the same wavelength.
A) photons with longer wavelengths have lower frequencies.
B) radio wave photons have shorter wavelengths than gamma-ray photons.
C) X-rays can be transmitted through the atmosphere around the world.
D) ultraviolet photons have less energy than visible photons.
E) all photons have the same wavelength.
photons with longer wavelengths have lower frequencies.
3
Which of the following lists different types of electromagnetic radiation in order from the shortest wavelength to the longest wavelength?
A) radio waves, infrared, visible, ultraviolet, X-rays
B) gamma rays, ultraviolet, visible, infrared, radio waves
C) gamma rays, X-rays, infrared, visible, ultraviolet
D) X-rays, infrared, visible, ultraviolet, radio waves
E) radio waves, ultraviolet, visible, infrared, gamma rays
A) radio waves, infrared, visible, ultraviolet, X-rays
B) gamma rays, ultraviolet, visible, infrared, radio waves
C) gamma rays, X-rays, infrared, visible, ultraviolet
D) X-rays, infrared, visible, ultraviolet, radio waves
E) radio waves, ultraviolet, visible, infrared, gamma rays
gamma rays, ultraviolet, visible, infrared, radio waves
4
Light has aspects of
A) only a wave.
B) only a particle.
C) both a particle and a wave.
D) neither a particle nor a wave.
E) none of these are correct.
A) only a wave.
B) only a particle.
C) both a particle and a wave.
D) neither a particle nor a wave.
E) none of these are correct.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
5
The speed of light in a vacuum is
A) 300,000 m/s.
B) 300,000 mph.
C) 300,000 km/s.
D) 300,000,000 mph.
E) infinite.
A) 300,000 m/s.
B) 300,000 mph.
C) 300,000 km/s.
D) 300,000,000 mph.
E) infinite.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
6
The term quantum refers to
A) anything that is very small.
B) a way of grouping small things together for mathematical convenience.
C) a single, smallest possible unit of some quantity.
D) uncertain, theoretical concepts.
E) something that behaves randomly.
A) anything that is very small.
B) a way of grouping small things together for mathematical convenience.
C) a single, smallest possible unit of some quantity.
D) uncertain, theoretical concepts.
E) something that behaves randomly.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
7
Saying that something is quantized means that it
A) is a wave.
B) can only be observed using a telescope.
C) travels at the speed of light.
D) can only have discrete quantities.
E) is smaller than an atom.
A) is a wave.
B) can only be observed using a telescope.
C) travels at the speed of light.
D) can only have discrete quantities.
E) is smaller than an atom.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
8
The amplitude of a wave is
A) the total distance a wave travels
B) a measure of the wave's velocity.
C) the number of peaks that pass per second.
D) the maximum height of the wave above zero.
E) the distance between one peak value and the next peak.
A) the total distance a wave travels
B) a measure of the wave's velocity.
C) the number of peaks that pass per second.
D) the maximum height of the wave above zero.
E) the distance between one peak value and the next peak.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
9
What is the difference between visible light and X-rays?
A) Speed; X-rays go faster than visible light.
B) Speed; X-rays go slower than visible light.
C) Wavelength; X-rays have a shorter wavelength than visible light.
D) Wavelength; X-rays have a longer wavelength than visible light.
E) X-rays are made up of particles, whereas visible light is made up of waves.
A) Speed; X-rays go faster than visible light.
B) Speed; X-rays go slower than visible light.
C) Wavelength; X-rays have a shorter wavelength than visible light.
D) Wavelength; X-rays have a longer wavelength than visible light.
E) X-rays are made up of particles, whereas visible light is made up of waves.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
10
Which of the following photons carries the smallest amount of energy?
A) a blue photon of the visible spectrum, whose wavelength is 450 nm
B) an infrared photon, whose wavelength is 10-5 m
C) a red photon in the visible spectrum, whose wavelength is 700 nm
D) a microwave photon, whose wavelength is 10-2 m
E) an ultraviolet photon, whose wavelength is 300 nm
A) a blue photon of the visible spectrum, whose wavelength is 450 nm
B) an infrared photon, whose wavelength is 10-5 m
C) a red photon in the visible spectrum, whose wavelength is 700 nm
D) a microwave photon, whose wavelength is 10-2 m
E) an ultraviolet photon, whose wavelength is 300 nm

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
11
How do the wavelength and frequency of red light compare to the wavelength and frequency of blue light?
A) Red light has a longer wavelength and higher frequency than blue light.
B) Red light has a longer wavelength and lower frequency than blue light.
C) Red light has a shorter wavelength and higher frequency than blue light.
D) Red light has a shorter wavelength and lower frequency than blue light.
E) Red light and blue light will have the same frequencies and wavelengths.
A) Red light has a longer wavelength and higher frequency than blue light.
B) Red light has a longer wavelength and lower frequency than blue light.
C) Red light has a shorter wavelength and higher frequency than blue light.
D) Red light has a shorter wavelength and lower frequency than blue light.
E) Red light and blue light will have the same frequencies and wavelengths.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
12
The color of visible light is determined by its
A) speed.
B) wavelength.
C) mass.
D) distance from you.
E) size.
A) speed.
B) wavelength.
C) mass.
D) distance from you.
E) size.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
13
Which formula denotes how the speed of light is related to its wavelength and frequency?
A) c = f
B) c = /f
C) c =f /
D) c = 1/ f
E) There is no relationship between wavelength and frequency.
A) c = f
B) c = /f
C) c =f /
D) c = 1/ f
E) There is no relationship between wavelength and frequency.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
14
The wavelength of a wave is
A) the total distance a wave travels
B) a measure of the wave's velocity.
C) the number of peaks that pass per second.
D) the maximum height of the wave above zero.
E) the distance between one peak value and the next peak.
A) the total distance a wave travels
B) a measure of the wave's velocity.
C) the number of peaks that pass per second.
D) the maximum height of the wave above zero.
E) the distance between one peak value and the next peak.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
15
If the wavelength of a beam of light were to double, how would that affect its frequency?
A) The frequency would be four times higher.
B) The frequency would be two times higher.
C) The frequency would be two times lower.
D) The frequency would be four times lower.
E) There is no relationship between wavelength and frequency.
A) The frequency would be four times higher.
B) The frequency would be two times higher.
C) The frequency would be two times lower.
D) The frequency would be four times lower.
E) There is no relationship between wavelength and frequency.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
16
When talking about a wave, what does the term medium refer to?
A) the size of an object
B) the substance through which the wave travels
C) the brightness level
D) the vacuum
E) the wavelength range of that wave
A) the size of an object
B) the substance through which the wave travels
C) the brightness level
D) the vacuum
E) the wavelength range of that wave

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
17
Radio waves have wavelengths of
A) less than 0.1 nanometer.
B) between 0.1 nanometer and 40 nanometers.
C) between 40 nanometers and 380 nanometers.
D) between 1,000 nanometers and 500 microns.
E) more than a few centimeters.
A) less than 0.1 nanometer.
B) between 0.1 nanometer and 40 nanometers.
C) between 40 nanometers and 380 nanometers.
D) between 1,000 nanometers and 500 microns.
E) more than a few centimeters.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
18
How does the speed of light traveling through a medium (such as air or glass) compare to the speed of light in a vacuum?
A) It is the same as the speed of light in a vacuum.
B) It is always less than the speed of light in a vacuum.
C) It is always greater than the speed of light in a vacuum.
D) Sometimes it is greater than the speed of light in a vacuum and sometimes it is less, depending on the medium.
E) Light can't travel through a medium; it only can go through a vacuum.
A) It is the same as the speed of light in a vacuum.
B) It is always less than the speed of light in a vacuum.
C) It is always greater than the speed of light in a vacuum.
D) Sometimes it is greater than the speed of light in a vacuum and sometimes it is less, depending on the medium.
E) Light can't travel through a medium; it only can go through a vacuum.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
19
A nanometer is ________ of a meter.
A) 1/100
B) 1/1,000
C) 1/1,000,000
D) 1/1,000,000,000
E) 1/1,000,000,000,000
A) 1/100
B) 1/1,000
C) 1/1,000,000
D) 1/1,000,000,000
E) 1/1,000,000,000,000

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
20
________ measured the speed of light using the timing of Jupiter's moon.
A) Galileo
B) Newton
C) Kepler
D) Rømer
E) Einstein
A) Galileo
B) Newton
C) Kepler
D) Rømer
E) Einstein

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
21
A certain light wave has a frequency of 3 *108 Hz.What is its wavelength?
A) 500 nanometers
B) 10 nanometers
C) 100 microns
D) 1.0 meter
E) 3 * 108 meters
A) 500 nanometers
B) 10 nanometers
C) 100 microns
D) 1.0 meter
E) 3 * 108 meters

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
22
In the energy level diagram shown in the figure below, the electron is excited to the E2 energy level.If the atom absorbs a photon with the exact wavelength to move the electron to another energy level, which energy level would correspond to the incoming photon with the largest wavelength? 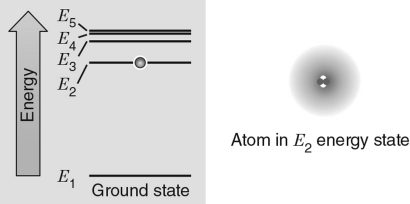
A) E1
B) E2
C) E3
D) E4
E) E5

A) E1
B) E2
C) E3
D) E4
E) E5

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
23
If the frequency of a beam of light were to increase, its period would ________ and its wavelength would ________.
A) decrease; increase
B) increase; decrease
C) increase; increase
D) decrease; decrease
E) stay the same; stay the same
A) decrease; increase
B) increase; decrease
C) increase; increase
D) decrease; decrease
E) stay the same; stay the same

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
24
Which of these objects would emit an absorption spectrum?
A) an incandescent lightbulb
B) a fluorescent lightbulb
C) an isolated hot gas cloud
D) a hot, solid object
E) a thin, cool gas cloud that lies in front of a hotter blackbody
A) an incandescent lightbulb
B) a fluorescent lightbulb
C) an isolated hot gas cloud
D) a hot, solid object
E) a thin, cool gas cloud that lies in front of a hotter blackbody

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
25
The n = 5 electronic energy level in a hydrogen atom is 1.5 *10-19 J higher than the n = 3 level.If an electron moves from the n = 5 level to the n = 3 level, then a photon of wavelength
A) 1.3 nm, which is in the ultraviolet region, is emitted.
B) 1.3 nm, which is in the ultraviolet region, is absorbed.
C) 1,300 nm, which is in the infrared region, is absorbed.
D) 1,300 nm, which is in the infrared region, is emitted.
E) No light will be absorbed or emitted.
A) 1.3 nm, which is in the ultraviolet region, is emitted.
B) 1.3 nm, which is in the ultraviolet region, is absorbed.
C) 1,300 nm, which is in the infrared region, is absorbed.
D) 1,300 nm, which is in the infrared region, is emitted.
E) No light will be absorbed or emitted.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
26
When an electron moves from a higher energy level in an atom to a lower energy level,
A) the atom is ionized.
B) a continuous spectrum is emitted.
C) a photon is emitted.
D) a photon is absorbed.
E) the electron loses mass.
A) the atom is ionized.
B) a continuous spectrum is emitted.
C) a photon is emitted.
D) a photon is absorbed.
E) the electron loses mass.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
27
The figure below illustrates a stellar spectrum.The dip in the data near 650 nm corresponds most closely with which of the following? 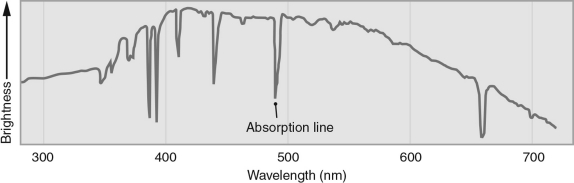
A) sodium emission
B) sodium absorption
C) hydrogen emission
D) hydrogen absorption
E) iron absorption

A) sodium emission
B) sodium absorption
C) hydrogen emission
D) hydrogen absorption
E) iron absorption

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
28
In the energy level diagram shown in the figure below, the electron is excited to the E4 energy level.If the electron transitions to an energy level giving off a photon, which level would produce a photon with the largest frequency? 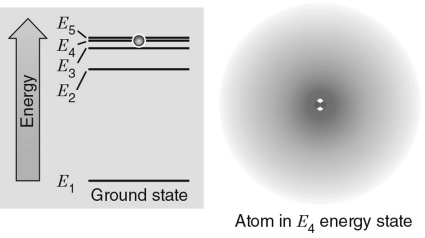
A) E1
B) E2
C) E3
D) E4
E) E5

A) E1
B) E2
C) E3
D) E4
E) E5

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
29
The frequency of a wave is
A) the total distance a wave travels
B) a measure of the wave's velocity.
C) the number of peaks that pass per second.
D) the maximum height of the wave above zero.
E) the distance between one peak value and the next peak.
A) the total distance a wave travels
B) a measure of the wave's velocity.
C) the number of peaks that pass per second.
D) the maximum height of the wave above zero.
E) the distance between one peak value and the next peak.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
30
Light with a wavelength of 600 nm has a frequency of
A) 2 *105 Hz
B) 5 * 107 Hz
C) 2 *1010 Hz
D) 5 * 1012 Hz
E) 5 * 1014 Hz
A) 2 *105 Hz
B) 5 * 107 Hz
C) 2 *1010 Hz
D) 5 * 1012 Hz
E) 5 * 1014 Hz

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
31
In the energy level diagram shown in the figure below, the electron is excited to the E4 energy level.If the electron transitions to an energy level giving off a photon, which level would produce a photon with the largest wavelength? 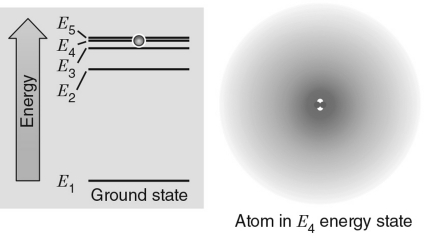
A) E1
B) E2
C) E3
D) E4
E) E5

A) E1
B) E2
C) E3
D) E4
E) E5

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
32
In the quantum mechanical view of the atom, electrons are often depicted as
A) a cloud that is centered on the nucleus.
B) a particle orbiting the nucleus.
C) free to orbit at any distance from the nucleus.
D) a particle inside the nucleus.
E) nothing, electrons are not part of an atom.
A) a cloud that is centered on the nucleus.
B) a particle orbiting the nucleus.
C) free to orbit at any distance from the nucleus.
D) a particle inside the nucleus.
E) nothing, electrons are not part of an atom.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
33
As wavelength increases, the energy of a photon ________ and its frequency ________.
A) increases; decreases
B) increases; increases
C) decreases; decreases
D) decreases; increases
E) decreases; does not change.
A) increases; decreases
B) increases; increases
C) decreases; decreases
D) decreases; increases
E) decreases; does not change.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
34
The Doppler shift can be used to determine the ________ of an object.
A) energy
B) temperature
C) radial velocity
D) color
E) three-dimensional velocity
A) energy
B) temperature
C) radial velocity
D) color
E) three-dimensional velocity

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
35
If you observe the spectrum of an isolated hot cloud of gas, you will see
A) an absorption spectrum.
B) a continuous spectrum.
C) an emission spectrum.
D) a rainbow spectrum.
E) a dark spectrum.
A) an absorption spectrum.
B) a continuous spectrum.
C) an emission spectrum.
D) a rainbow spectrum.
E) a dark spectrum.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
36
In the energy level diagram shown in the figure below, the electron is excited to the E4 energy level.If the electron transitions to an energy level giving off a photon, which level would produce a photon with the largest energy? 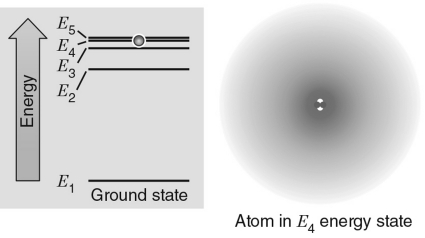
A) E1
B) E2
C) E3
D) E4
E) E5

A) E1
B) E2
C) E3
D) E4
E) E5

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
37
A red photon has a wavelength of 650 nm.An ultraviolet photon has a wavelength of 250 nm.The energy of an ultraviolet photon is ________ the energy of a red photon.
A) 2.6 times larger than
B) 6.8 times larger than
C) 2.6 times smaller than
D) 6.8 times smaller than
E) the same as
A) 2.6 times larger than
B) 6.8 times larger than
C) 2.6 times smaller than
D) 6.8 times smaller than
E) the same as

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
38
In the energy level diagram shown in the figure below, the electron is excited to the E2 energy level.If the atom absorbs a photon with the exact frequency to move the electron to another energy level, which energy level would correspond to the incoming photon with the largest frequency? 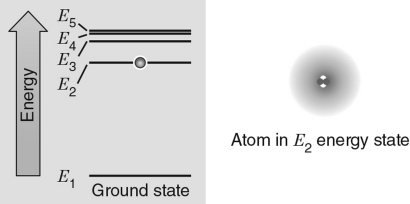
A) E1
B) E2
C) E3
D) E4
E) E5

A) E1
B) E2
C) E3
D) E4
E) E5

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
39
If you observe the spectrum of a star, you will see
A) an absorption spectrum.
B) a continuous spectrum.
C) an emission spectrum.
D) a rainbow spectrum.
E) a dark spectrum.
A) an absorption spectrum.
B) a continuous spectrum.
C) an emission spectrum.
D) a rainbow spectrum.
E) a dark spectrum.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
40
Why is a neutral iron atom a different element from a neutral carbon atom?
A) A carbon atom has fewer neutrons in its nucleus than an iron atom.
B) An iron atom has more protons in its nucleus than a carbon atom.
C) An iron atom has more electrons than a carbon atom.
D) A carbon atom is bigger than an iron atom.
E) None of these is correct.
A) A carbon atom has fewer neutrons in its nucleus than an iron atom.
B) An iron atom has more protons in its nucleus than a carbon atom.
C) An iron atom has more electrons than a carbon atom.
D) A carbon atom is bigger than an iron atom.
E) None of these is correct.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
41
Compare two blackbody objects, one at 200 K and one at 400 K.How much larger is the flux from the 400 K object compared to the flux from the 200 K object?
A) 2 times larger
B) 4 times larger
C) 8 times larger
D) 16 times larger
E) They have the same flux.
A) 2 times larger
B) 4 times larger
C) 8 times larger
D) 16 times larger
E) They have the same flux.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
42
You observe the spectrum of two stars.Star A has an emission line from a known element at 600 nm.Star B has emission lines from the same atom, but the emission line is occurring at 650 nm.One possible explanation for this observation is that star A is
A) cooler than star B.
B) farther away from us than star B.
C) moving toward us faster than star B.
D) made of different elements than star B.
E) larger than star B.
A) cooler than star B.
B) farther away from us than star B.
C) moving toward us faster than star B.
D) made of different elements than star B.
E) larger than star B.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
43
If the quantity of something entering a system is equal to the quantity of that same thing leaving that system, we say the system is
A) quantized.
B) in equilibrium.
C) acting like a wave.
D) Doppler shifted.
E) dynamically unbalanced.
A) quantized.
B) in equilibrium.
C) acting like a wave.
D) Doppler shifted.
E) dynamically unbalanced.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
44
You record the spectrum of a star and find that a calcium absorption line has an observed wavelength of 394.0 nm.This calcium absorption line has a rest wavelength of 393.3 nm.What is the approximate radial velocity of this star?
A) 5,000 km/s
B) 500 km/s
C) 50 km/s
D) 5 km/s
E) 0.5 km/s
A) 5,000 km/s
B) 500 km/s
C) 50 km/s
D) 5 km/s
E) 0.5 km/s

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
45
If you find that the hydrogen alpha line in a star's spectrum occurs at a wavelength of 656.45 nm, what is the star's approximate radial velocity? Note that the rest wavelength of this line is 656.30 nm.
A) 150 km/s away from you
B) 150 km/s toward you
C) 350 km/s toward you
D) 70 km/s away from you
E) 70 km/s toward you
A) 150 km/s away from you
B) 150 km/s toward you
C) 350 km/s toward you
D) 70 km/s away from you
E) 70 km/s toward you

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
46
You observe a red star and a blue star and are able to determine that they are the same size.Which star has a higher surface temperature, and which star is more luminous?
A) The red star has a higher surface temperature and is more luminous.
B) The red star has a higher surface temperature, and the blue star is more luminous.
C) The blue star has a higher surface temperature and is more luminous.
D) The blue star has a higher surface temperature, and the red star is more luminous.
E) They have the same luminosities and temperatures.
A) The red star has a higher surface temperature and is more luminous.
B) The red star has a higher surface temperature, and the blue star is more luminous.
C) The blue star has a higher surface temperature and is more luminous.
D) The blue star has a higher surface temperature, and the red star is more luminous.
E) They have the same luminosities and temperatures.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
47
The Kelvin temperature scale is used in astronomy because
A) at 0 K an object has zero thermal energy.
B) water freezes at 0 K.
C) water boils at 100 K.
D) hydrogen freezes at 0 K.
E) the highest temperature possible is 1000 K.
A) at 0 K an object has zero thermal energy.
B) water freezes at 0 K.
C) water boils at 100 K.
D) hydrogen freezes at 0 K.
E) the highest temperature possible is 1000 K.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
48
At what temperature does water freeze?
A) 0 K
B) 32 K
C) 100 K
D) 273 K
E) 373 K
A) 0 K
B) 32 K
C) 100 K
D) 273 K
E) 373 K

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
49
Why do some stars in the sky appear blue, whereas other stars appear red?
A) The red stars have higher surface temperatures than the blue stars.
B) The blue stars have higher surface temperatures than the red stars.
C) The blue stars are closer to us than the red stars.
D) The red stars are closer to us than the blue stars.
E) The blue stars are moving toward us, while red stars are moving away from us.
A) The red stars have higher surface temperatures than the blue stars.
B) The blue stars have higher surface temperatures than the red stars.
C) The blue stars are closer to us than the red stars.
D) The red stars are closer to us than the blue stars.
E) The blue stars are moving toward us, while red stars are moving away from us.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
50
You are driving on the freeway when a police officer records a shift of -7 nm when he or she measures your speed with a radar gun that operates at a wavelength of 0.1 m.How fast were you going?
A) 43 mph
B) 83 mph
C) 21 mph
D) 65 mph
E) 47 mph
A) 43 mph
B) 83 mph
C) 21 mph
D) 65 mph
E) 47 mph

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
51
In the figure below, which blackbody spectrum corresponds to the object that would appear white to the human eye? 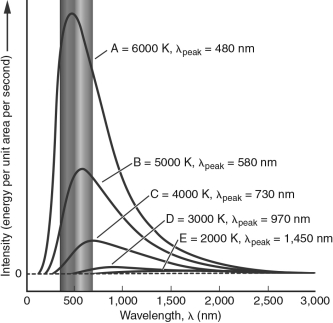
A) A
B) B
C) C
D) D
E) E

A) A
B) B
C) C
D) D
E) E

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
52
As an object's temperature increases, the atoms inside that object
A) gain electrons.
B) increase in mass.
C) move at higher velocities.
D) emit less red light.
E) get larger.
A) gain electrons.
B) increase in mass.
C) move at higher velocities.
D) emit less red light.
E) get larger.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
53
What does it mean to say that an object is in thermal equilibrium?
A) It isn't absorbing any energy.
B) It isn't radiating any energy.
C) It is radiating more energy than it is absorbing.
D) It is absorbing more energy than it is radiating.
E) It is absorbing the same amount of energy that it is radiating.
A) It isn't absorbing any energy.
B) It isn't radiating any energy.
C) It is radiating more energy than it is absorbing.
D) It is absorbing more energy than it is radiating.
E) It is absorbing the same amount of energy that it is radiating.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
54
Which of these stars would have the biggest redshift?
A) a star moving at low speed toward you
B) a star moving at high speed toward you
C) a star moving at low speed away from you
D) a star moving at high speed away from you
E) a star that is not moving away from you or toward you
A) a star moving at low speed toward you
B) a star moving at high speed toward you
C) a star moving at low speed away from you
D) a star moving at high speed away from you
E) a star that is not moving away from you or toward you

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
55
In the figure below, which blackbody spectrum corresponds to the object that would appear the reddest to the human eye? 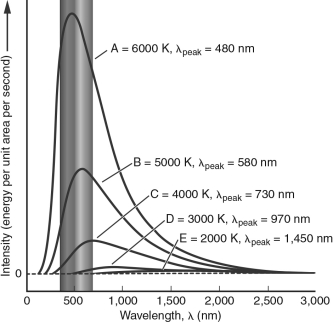
A) A
B) B
C) C
D) D
E) E

A) A
B) B
C) C
D) D
E) E

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
56
Consider an incandescent lightbulb.If you wanted to turn a 10-W lightbulb into a 100-W lightbulb, how would you change the temperature of the filament inside the bulb?
A) Raise its temperature by a factor of 3.2.
B) Raise its temperature by a factor of 1.8.
C) Raise its temperature by a factor of 10.
D) Lower its temperature by a factor of 2.6.
E) Lower its temperature by a factor of 5.4.
A) Raise its temperature by a factor of 3.2.
B) Raise its temperature by a factor of 1.8.
C) Raise its temperature by a factor of 10.
D) Lower its temperature by a factor of 2.6.
E) Lower its temperature by a factor of 5.4.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
57
As a blackbody's temperature increases, it also becomes ________ and ________.
A) more luminous; redder
B) more luminous; bluer
C) less luminous; redder
D) less luminous; bluer
E) more luminous; stays the same color
A) more luminous; redder
B) more luminous; bluer
C) less luminous; redder
D) less luminous; bluer
E) more luminous; stays the same color

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
58
A spaceship is traveling at a speed of 15,000 km/s from planet "B" toward planet "A." The spaceship sends out a signal with a wavelength of 4 m.If astronomers living on planets A and B measure the radio waves coming from the spaceship, what wavelengths will they measure?
A) Planet A measures 6 m, and planet B measures 2 m.
B) Planet A measures 2 m, and planet B measures 6 m.
C) Planet A measures 4.2 m, and planet B measures 3.8 m.
D) Planet A measures 3.8 m, and planet B measures 4.2 m.
E) Both Planet A and planet B measure 4 m.
A) Planet A measures 6 m, and planet B measures 2 m.
B) Planet A measures 2 m, and planet B measures 6 m.
C) Planet A measures 4.2 m, and planet B measures 3.8 m.
D) Planet A measures 3.8 m, and planet B measures 4.2 m.
E) Both Planet A and planet B measure 4 m.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
59
In the figure below, which blackbody spectrum corresponds to the object with the highest temperature? 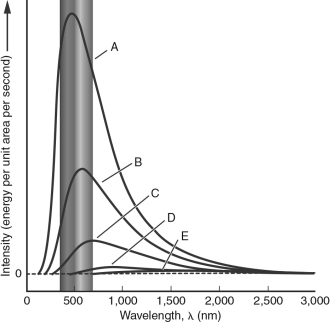
A) A
B) B
C) C
D) D
E) E

A) A
B) B
C) C
D) D
E) E

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
60
A spaceship is traveling toward Earth while giving off a constant radio signal with a wavelength of 1 meter (m).What will the signal look like to people on Earth?
A) a signal with a wavelength less than 1 m
B) a signal with a wavelength more than 1 m
C) a signal moving faster than the speed of light
D) a signal moving slower than the speed of light
E) a signal with a wavelength of 1 m, moving the normal speed of light
A) a signal with a wavelength less than 1 m
B) a signal with a wavelength more than 1 m
C) a signal moving faster than the speed of light
D) a signal moving slower than the speed of light
E) a signal with a wavelength of 1 m, moving the normal speed of light

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
61
The average red giant is about 1,000 times more luminous than the average main-sequence star.If both kinds of stars have about the same brightness in our night sky, how much farther away are the red giants compared to the main-sequence stars?
A) 32 times farther
B) 1,000 times farther
C) 65 times farther
D) 5.6 times farther
E) The red giants and main-sequence stars are approximately the same distance away.
A) 32 times farther
B) 1,000 times farther
C) 65 times farther
D) 5.6 times farther
E) The red giants and main-sequence stars are approximately the same distance away.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
62
Describe, in your own words, why electrons cannot orbit the nucleus like the planets orbit the Sun.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
63
Why do we see black lines in an absorption spectrum if the absorbed photons are (almost) instantaneously reemitted by the atoms in the cloud?

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
64
Which of these planets would be expected to have the highest average temperature?
A) a light-colored planet close to its star
B) a dark-colored planet close to its star
C) a light-colored planet far from its star
D) a dark-colored planet far from its star
E) There is not enough information to know which would be the hottest.
A) a light-colored planet close to its star
B) a dark-colored planet close to its star
C) a light-colored planet far from its star
D) a dark-colored planet far from its star
E) There is not enough information to know which would be the hottest.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
65
If a star has a peak wavelength of 290 nm, what is its surface temperature?
A) 1000 K
B) 2000 K
C) 5000 K
D) 10,000 K
E) 100,000 K
A) 1000 K
B) 2000 K
C) 5000 K
D) 10,000 K
E) 100,000 K

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
66
A spaceship approaches Earth at 0.9 times the speed of light and shines a powerful searchlight onto Earth.How fast will the photons from this searchlight be moving when they hit Earth?

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
67
What different processes produce the patterns of light that can be viewed as: continuous spectra; emission spectra; and absorption spectra?

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
68
The difference in energy between the n = 2 and n =1 electronic energy levels in the hydrogen atom is 1.6 *10-18 J.If an electron moves from the n = 1 level to the n=2 level, will a photon be emitted or absorbed? What will its energy be, and what type of electromagnetic radiation is it? Use the electromagnetic spectrum shown in the figure below to answer this question. 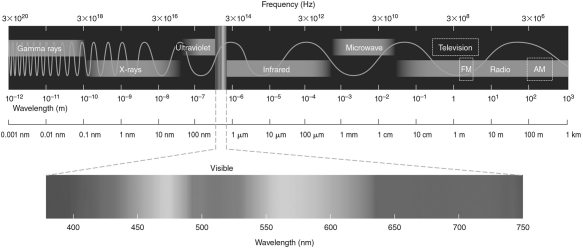


Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
69
Star C and star D have the same luminosity.Star C is twice as far away from Earth as star D.How do the brightness levels of stars C and D compare?
A) Star C appears four times as bright as star D.
B) Star C appears twice as bright as star D.
C) Star D appears twice as bright as star C.
D) Star D appears four times as bright as star C.
E) Stars C and D appear equally bright.
A) Star C appears four times as bright as star D.
B) Star C appears twice as bright as star D.
C) Star D appears twice as bright as star C.
D) Star D appears four times as bright as star C.
E) Stars C and D appear equally bright.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
70
If the Sun's luminosity were twice its actual value, Earth's temperature would
A) more than double.
B) double.
C) increase, but be less than double.
D) not be different.
E) decrease a small amount.
A) more than double.
B) double.
C) increase, but be less than double.
D) not be different.
E) decrease a small amount.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
71
Compare the differences between a photon of red light and a photon of blue light.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
72
At what peak wavelength does your body radiate the most given that your temperature is approximately that of Earth, which is 300 K?
A) 10-5 m
B) 10-3 m
C) 10-2 m
D) 10 m
E) 1,000 m
A) 10-5 m
B) 10-3 m
C) 10-2 m
D) 10 m
E) 1,000 m

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
73
The first five energy levels of hydrogen are E1 = 0 eV, E2 = 10.2 eV, E3 =12.1 eV, E4 =12.7 eV, and E5 = 13.1 eV.If the electron is in the n = 4 level, what energies can a single emitted photon have?

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
74
If a lightbulb is moved twice as far away, its luminosity will be
A) doubled.
B) quadrupled.
C) halved.
D) 1/4 the original.
E) the same.
A) doubled.
B) quadrupled.
C) halved.
D) 1/4 the original.
E) the same.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
75
How is the energy of a photon related to its frequency, wavelength, and speed?

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
76
How are atoms excited, and why do they become de-excited?

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
77
If Jupiter has a temperature of 165 K, at what wavelength does its spectrum peak? Use the electromagnetic spectrum in the figure below to answer this question. 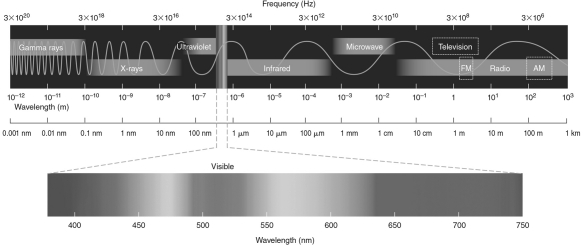
A) 18 nm-orange visible wavelengths
B) 1,800 mm-microwave wavelengths
C) 1,800 nm-infrared wavelengths
D) 18,000 nm-ultraviolet wavelengths
E) 18,000 nm-infrared wavelengths

A) 18 nm-orange visible wavelengths
B) 1,800 mm-microwave wavelengths
C) 1,800 nm-infrared wavelengths
D) 18,000 nm-ultraviolet wavelengths
E) 18,000 nm-infrared wavelengths

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
78
If the typical temperature of a red giant is 3000 K, at what wavelength is its radiation the brightest? Use the electromagnetic spectrum in the figure below to help you answer this question. 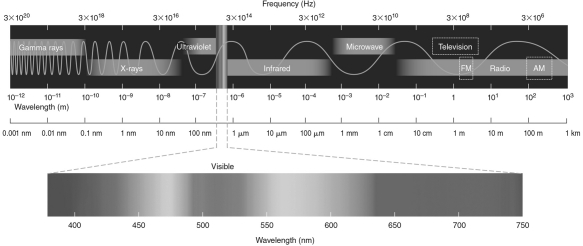
A) 1 m-infrared wavelengths
B) 1 m-red visible wavelengths
C) 20 m-infrared wavelengths
D) 20 m-red visible wavelengths
E) 700 m-red visible wavelengths

A) 1 m-infrared wavelengths
B) 1 m-red visible wavelengths
C) 20 m-infrared wavelengths
D) 20 m-red visible wavelengths
E) 700 m-red visible wavelengths

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
79
Star A and star B appear equally bright in the sky.Star A is twice as far away from Earth as star B.How do the luminosities of stars A and B compare?
A) Star A is twice as luminous as star B.
B) Star B is twice as luminous as star A.
C) Star A is four times as luminous as star B.
D) Star B is four times as luminous as star A.
E) Stars A and B have the same luminosity.
A) Star A is twice as luminous as star B.
B) Star B is twice as luminous as star A.
C) Star A is four times as luminous as star B.
D) Star B is four times as luminous as star A.
E) Stars A and B have the same luminosity.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
80
What is an electromagnetic wave?

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck



