Deck 14: Chemical Kinetics: Reactions in the Air We Breathe
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/164
Play
Full screen (f)
Deck 14: Chemical Kinetics: Reactions in the Air We Breathe
1
At what time of day are ozone concentrations highest during a photochemical smog event?
A) in the morning before rush hour
B) in the morning just after rush hour
C) midmorning
D) noon
E) midafternoon
A) in the morning before rush hour
B) in the morning just after rush hour
C) midmorning
D) noon
E) midafternoon
midafternoon
2
For the reaction 2A + 3B 4C + 5D, the rate of the reaction in terms of A would be written as ________
A) - 0A/ t.
B) -1/2 A/ t.
C) + A/ t.
D) +1/2 A/ t.
E) -2 A/ t.
A) - 0A/ t.
B) -1/2 A/ t.
C) + A/ t.
D) +1/2 A/ t.
E) -2 A/ t.
-1/2 A/ t.
3
One reaction that occurs in an automobile catalytic converter is the conversion of nitrogen monoxide to nitrogen and oxygen. How is the rate of this reaction related to the rate at which the concentration of a reactant or product changes?
2NO(g) N2(g) + O2(g)
I. Rate = [NO]/ t
II. Rate = - [NO]/ t
III. Rate = [N2]/ t
IV. Rate = [O2]/ t
A) I only
B) II only
C) III and IV only
D) I, III, and IV only
E) II, III, and IV only
2NO(g) N2(g) + O2(g)
I. Rate = [NO]/ t
II. Rate = - [NO]/ t
III. Rate = [N2]/ t
IV. Rate = [O2]/ t
A) I only
B) II only
C) III and IV only
D) I, III, and IV only
E) II, III, and IV only
III and IV only
4
The device in automobiles that has decreased NO and partially oxidized hydrocarbons from exhaust gases is termed a ________
A) catalytic intermediate.
B) photochemical inhibitor.
C) catalytic linker.
D) nitrogen monoxide reducer.
E) catalytic converter.
A) catalytic intermediate.
B) photochemical inhibitor.
C) catalytic linker.
D) nitrogen monoxide reducer.
E) catalytic converter.

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
5
Smog created by the interaction of sunlight with nitrogen oxides and volatile organic compounds is termed ________ smog.
A) photolytic
B) photochemical
C) photophysical
D) acid
E) sulfurous
A) photolytic
B) photochemical
C) photophysical
D) acid
E) sulfurous

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
6
The difference between an average rate and an instantaneous rate is ________
A) the average rate is taken over a longer time period.
B) the instantaneous rate is taken from the slope of the curve at a specific time.
C) They are not different if the time interval chosen is small enough.
D) All of the above are correct.
A) the average rate is taken over a longer time period.
B) the instantaneous rate is taken from the slope of the curve at a specific time.
C) They are not different if the time interval chosen is small enough.
D) All of the above are correct.

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
7
For the reaction 2A + 3B 4C + 5D, the rate of the reaction in terms of C would be written as ________
A) + C/ t.
B) +4 C/ t.
C) +1/4 C/ t.
D) -4 C/ t.
E) -1/4 C/ t.
A) + C/ t.
B) +4 C/ t.
C) +1/4 C/ t.
D) -4 C/ t.
E) -1/4 C/ t.

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
8
The greatest NO concentration is observed ________
A) in the morning before rush hour.
B) in the morning just after rush hour.
C) in midmorning.
D) at noon.
E) in midafternoon.
A) in the morning before rush hour.
B) in the morning just after rush hour.
C) in midmorning.
D) at noon.
E) in midafternoon.

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
9
Large cities often issue ozone advisories. At what time of year would an advisory be likely to occur most often?
A) winter
B) spring
C) summer
D) fall
E) No season should have more advisories than another.
A) winter
B) spring
C) summer
D) fall
E) No season should have more advisories than another.

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
10
When one chemical is a precursor of another chemical in a series of reactions, the concentrations of the two species are said to be ________
A) catalytic.
B) volatile.
C) coalescent.
D) linked.
E) intermediate.
A) catalytic.
B) volatile.
C) coalescent.
D) linked.
E) intermediate.

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
11
For the reaction 2A + 3B 4C + 5D, the rate of the reaction in terms of B would be written as ________
A) - B/ t.
B) + B/ t.
C) -1/3 B/ t.
D) +1/3 B/ t.
E) -3 B/ t.
A) - B/ t.
B) + B/ t.
C) -1/3 B/ t.
D) +1/3 B/ t.
E) -3 B/ t.

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
12
Indicate which of the following compounds is a component of photochemical smog.
A) H2O
B) CO2
C) N2O
D) O3
E) CO
A) H2O
B) CO2
C) N2O
D) O3
E) CO

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
13
One reaction that occurs in an automobile catalytic converter is the conversion of nitrogen monoxide to nitrogen and oxygen. How could the rate of this reaction be expressed correctly in terms of the rate at which the concentration of a reactant or product changes?
2NO(g) N2(g) + O2(g)
A) Rate = [NO]/ t
B) Rate = - [NO]/ t
C) Rate =
[NO]/ t
D) Rate = -
[NO]/ t
E) Rate = 2 [NO]/ t
2NO(g) N2(g) + O2(g)
A) Rate = [NO]/ t
B) Rate = - [NO]/ t
C) Rate =
[NO]/ t
D) Rate = -
[NO]/ t
E) Rate = 2 [NO]/ t

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
14
NO2 contributes to the "brown haze" associated with photochemical smog events. At what time of day is NO2 concentration highest?
A) in the morning before rush hour
B) in the morning just after rush hour
C) midmorning
D) noon
E) midafternoon
A) in the morning before rush hour
B) in the morning just after rush hour
C) midmorning
D) noon
E) midafternoon

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
15
Which of the following is not important as a contributing factor to photochemical smog?
A) stagnant air
B) sunlight
C) lots of traffic
D) irrigation
E) cars without catalytic converters
A) stagnant air
B) sunlight
C) lots of traffic
D) irrigation
E) cars without catalytic converters

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
16
For the reaction 2A + 3B 4C + 5D, the rate of the reaction in terms of D would be written as ________
A) + D/ t.
B) +5 D/ t.
C) +1/5 D/ t.
D) -5 D/ t.
E) -1/5 D/ t.
A) + D/ t.
B) +5 D/ t.
C) +1/5 D/ t.
D) -5 D/ t.
E) -1/5 D/ t.

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
17
The order in which ozone, nitrogen monoxide, and nitrogen dioxide build up in the atmosphere over the course of the day is ________, then ________, then ________
A) NO; NO2; O3.
B) NO; O3; NO2.
C) NO2; NO; O3.
D) NO2; O3; NO.
E) O3; NO; NO2.
A) NO; NO2; O3.
B) NO; O3; NO2.
C) NO2; NO; O3.
D) NO2; O3; NO.
E) O3; NO; NO2.

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
18
NO2 concentrations during photochemical smog events often have two peaks, one in the early morning (8 A.M.) and another, smaller peak around 7 P.M. The second peak is due to ________
A) NO2 emissions produced during the evening rush hour.
B) the reaction of NO and O3.
C) the photochemical formation of O3.
D) automobile NO emissions reacting with oxygen.
E) All of the above.
A) NO2 emissions produced during the evening rush hour.
B) the reaction of NO and O3.
C) the photochemical formation of O3.
D) automobile NO emissions reacting with oxygen.
E) All of the above.

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
19
One reaction that occurs in an automobile catalytic converter is the conversion of nitrogen monoxide to nitrogen and oxygen. How could the rate of this reaction be expressed correctly in terms of the rate at which the concentration of a reactant or product changes?
2NO(g) N2(g) + O2(g)
A) Rate = [NO]/ t
B) Rate = [O2]/ t
C) Rate = 2 [O2]/ t
D) Rate = -2 [NO]/ t
E) Rate = 2 [NO]/ t
2NO(g) N2(g) + O2(g)
A) Rate = [NO]/ t
B) Rate = [O2]/ t
C) Rate = 2 [O2]/ t
D) Rate = -2 [NO]/ t
E) Rate = 2 [NO]/ t

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
20
Indicate which of the following compounds is not a component of photochemical smog.
A) O3
B) H2O
C) NO2
D) NO
E) organic molecules
A) O3
B) H2O
C) NO2
D) NO
E) organic molecules

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
21
A scientist conducts an experiment to determine the rate of the following reaction:
N2(g) + O2(g) 2NO(g)
If the initial concentration of NO was 0.00 M and the concentration of NO was 0.050 M after 0.100 s, what is the average rate of the reaction?
A) 0.500 M/s
B) 1.00 M/s
C) 5.00 M/s
D) 10.0 M/s
E) 0.250 M/s
N2(g) + O2(g) 2NO(g)
If the initial concentration of NO was 0.00 M and the concentration of NO was 0.050 M after 0.100 s, what is the average rate of the reaction?
A) 0.500 M/s
B) 1.00 M/s
C) 5.00 M/s
D) 10.0 M/s
E) 0.250 M/s

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
22
One reaction that occurs in an automobile catalytic converter is the conversion of nitrogen monoxide to nitrogen and oxygen. Which one of the following statements is correct about the rates at which the concentrations change as the reaction progresses?
2NO(g) N2(g) + O2(g)
A) - [NO]/ t increases while [N2]/ t decreases.
B) - [NO]/ t decreases while [N2]/ t increases.
C) Both - NO]/ t and [N2]/ t decrease.
D) Both - [NO]/ t and [N2]/ t increase.
E) The relationship between [NO]/ t and [N2]/ t depends on the reaction mechanism.
2NO(g) N2(g) + O2(g)
A) - [NO]/ t increases while [N2]/ t decreases.
B) - [NO]/ t decreases while [N2]/ t increases.
C) Both - NO]/ t and [N2]/ t decrease.
D) Both - [NO]/ t and [N2]/ t increase.
E) The relationship between [NO]/ t and [N2]/ t depends on the reaction mechanism.

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
23
The following graph shows the kinetics curves for the reaction of oxygen with hydrogen to form water:
O2(g) + 2H2(g) 2H2O(g). Which curve is hydrogen?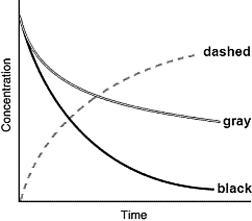
A) the dashed curve
B) the gray curve
C) the black curve
D) either the gray or the black curve
E) Any of these curves could be hydrogen.
O2(g) + 2H2(g) 2H2O(g). Which curve is hydrogen?

A) the dashed curve
B) the gray curve
C) the black curve
D) either the gray or the black curve
E) Any of these curves could be hydrogen.

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
24
HI dissociates to form I2 and H2:
2HI(g) H2(g) + I2(g)
If the concentration of HI changes at a rate of -0.45 M/s, what is the rate of appearance of I2(g)?
A) 0.90 M/s
B) 0.45 M/s
C) 0.23 M/s
D) 1.00 M/s
E) 0.13 M/s
2HI(g) H2(g) + I2(g)
If the concentration of HI changes at a rate of -0.45 M/s, what is the rate of appearance of I2(g)?
A) 0.90 M/s
B) 0.45 M/s
C) 0.23 M/s
D) 1.00 M/s
E) 0.13 M/s

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
25
A scientist conducts an experiment to determine the rate of the following reaction:
N2(g) + O2(g) 2NO(g)
If the initial concentration of N2 was 0.500 M and the concentration of N2 was 0.450 M after
0)100 s, what is the average rate of the reaction?
A) 0.500 M/s
B) 1.00 M/s
C) 5.00 M/s
D) 10.0 M/s
E) 0.250 M/s
N2(g) + O2(g) 2NO(g)
If the initial concentration of N2 was 0.500 M and the concentration of N2 was 0.450 M after
0)100 s, what is the average rate of the reaction?
A) 0.500 M/s
B) 1.00 M/s
C) 5.00 M/s
D) 10.0 M/s
E) 0.250 M/s

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
26
One reaction that occurs in an automobile catalytic converter is the conversion of carbon monoxide to carbon dioxide. How could the rate of this reaction be expressed correctly in terms of the rate at which the concentration of a reactant or product changes?
2CO(g) + O2(g) 2CO2(g)
A) Rate = CO]/ t
B) Rate = - [CO]/ t
C) Rate = O2]/ t
D) Rate = -
[CO]/ t
E) Rate = 2 [CO2]/ t
2CO(g) + O2(g) 2CO2(g)
A) Rate = CO]/ t
B) Rate = - [CO]/ t
C) Rate = O2]/ t
D) Rate = -
[CO]/ t
E) Rate = 2 [CO2]/ t

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
27
The disappearance of HI in the reaction 2HI(g) I2(g) + H2(g) is shown in the following figure. What is the instantaneous rate of this reaction at t = 5 s if the instantaneous rate of change of [HI] is 0.02 M/s? ![<strong>The disappearance of HI in the reaction 2HI(g) \rightarrow I<sub>2</sub>(g) + H<sub>2</sub>(g) is shown in the following figure. What is the instantaneous rate of this reaction at t = 5 s if the instantaneous rate of change of [HI] is 0.02 M/s? </strong> A) 0.2 M/s B) 0.01 M/s C) 0.02 M/s D) 0.100 M/s E) 0.04 M/s](https://d2lvgg3v3hfg70.cloudfront.net/TB3834/11eb0df4_173c_96b4_9431_2555c5696a90_TB3834_00.jpg)
A) 0.2 M/s
B) 0.01 M/s
C) 0.02 M/s
D) 0.100 M/s
E) 0.04 M/s
![<strong>The disappearance of HI in the reaction 2HI(g) \rightarrow I<sub>2</sub>(g) + H<sub>2</sub>(g) is shown in the following figure. What is the instantaneous rate of this reaction at t = 5 s if the instantaneous rate of change of [HI] is 0.02 M/s? </strong> A) 0.2 M/s B) 0.01 M/s C) 0.02 M/s D) 0.100 M/s E) 0.04 M/s](https://d2lvgg3v3hfg70.cloudfront.net/TB3834/11eb0df4_173c_96b4_9431_2555c5696a90_TB3834_00.jpg)
A) 0.2 M/s
B) 0.01 M/s
C) 0.02 M/s
D) 0.100 M/s
E) 0.04 M/s

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
28
One reaction that occurs in an automobile catalytic converter is the conversion of carbon monoxide to carbon dioxide. Examine the statements A-C: if one is incorrect, identify it. Otherwise, select response D or E as appropriate.
2CO(g) + O2(g) 2CO2(g)
A)(- CO]/ t = [CO2]/ t)
B) ( [CO]/F t = 2 [O2]/ t
C)( [O2]/ t = F [CO2]/ t)
D)Statements A-C are all correct.
E)More than one of the statements A-C is incorrect.
2CO(g) + O2(g) 2CO2(g)
A)(- CO]/ t = [CO2]/ t)
B) ( [CO]/F t = 2 [O2]/ t
C)( [O2]/ t = F [CO2]/ t)
D)Statements A-C are all correct.
E)More than one of the statements A-C is incorrect.

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
29
If the rate of appearance of O2 in the reaction:
2O3(g) 3O2(g)
Is 0.250 M/s over the first 5.50 s, how much oxygen will form during this time?
A) 1.38 M
B) 4.13 M
C) 0.69 M
D) 0.25 M
E) 0.46 M
2O3(g) 3O2(g)
Is 0.250 M/s over the first 5.50 s, how much oxygen will form during this time?
A) 1.38 M
B) 4.13 M
C) 0.69 M
D) 0.25 M
E) 0.46 M

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
30
Ammonia gas (NH3) is produced from hydrogen and nitrogen gas according to the following reaction:
3H2(g) + N2(g) 2NH3(g)
If the rate of production of ammonia is R(NH3), what is the rate of loss of hydrogen and nitrogen gas, respectively?
A) -R(H2) = 2/3 R(NH3); -R(N2) = 1/2 R(NH3)
B) -R(H2) = 3/2 R(NH3); -R(N2) = 2 R(NH3)
C) -R(H2) = 3/2 R(NH3); -R(N2) = 1/2 R(NH3)
D) -R(H2) = 2/3 R(NH3); -R(N2) = 2 R(NH3)
E) -R(H2) = R(NH3); -R(N2) = R(NH3)
3H2(g) + N2(g) 2NH3(g)
If the rate of production of ammonia is R(NH3), what is the rate of loss of hydrogen and nitrogen gas, respectively?
A) -R(H2) = 2/3 R(NH3); -R(N2) = 1/2 R(NH3)
B) -R(H2) = 3/2 R(NH3); -R(N2) = 2 R(NH3)
C) -R(H2) = 3/2 R(NH3); -R(N2) = 1/2 R(NH3)
D) -R(H2) = 2/3 R(NH3); -R(N2) = 2 R(NH3)
E) -R(H2) = R(NH3); -R(N2) = R(NH3)

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
31
A scientist conducts an experiment to determine the rate of NO formation in the reaction:
N2(g) + O2(g) 2NO(g)
If the initial concentration of N2 was 0.500 M and the concentration of N2 was 0.450 M after
0)100 s, what is the average rate of NO formation?
A) 0.500 M/s
B) 1.00 M/s
C) 5.00 M/s
D) 10.0 M/s
E) 0.250 M/s
N2(g) + O2(g) 2NO(g)
If the initial concentration of N2 was 0.500 M and the concentration of N2 was 0.450 M after
0)100 s, what is the average rate of NO formation?
A) 0.500 M/s
B) 1.00 M/s
C) 5.00 M/s
D) 10.0 M/s
E) 0.250 M/s

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
32
Which of the following does the initial rate of a chemical reaction depend on?
I.Concentration of reactants
II.Concentration of products
III.Temperature
A) I
B) III
C) II
D) Both I and III
E) I, II, and III
I.Concentration of reactants
II.Concentration of products
III.Temperature
A) I
B) III
C) II
D) Both I and III
E) I, II, and III

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
33
Which of the following statements is true about the instantaneous rate for the chemical reaction
A B as the reaction progresses?
A) - A/ t increases while B/ t decreases
B) - A/ t decreases while B/ t increases
C) both - A/ t and B/ t decrease
D) both - A/ t and B/ t increase
E) The answer will vary depending on the reaction mechanism.
A B as the reaction progresses?
A) - A/ t increases while B/ t decreases
B) - A/ t decreases while B/ t increases
C) both - A/ t and B/ t decrease
D) both - A/ t and B/ t increase
E) The answer will vary depending on the reaction mechanism.

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
34
If the rate of formation of ammonia is 0.345 M/s, what is the rate of disappearance of N2?
N2(g) + 3H2(g) 2NH3(g)
A) 0.173 M/s
B) 0.345 M/s
C) 0.690 M/s
D) 245 M/s
E) 0.518 M/s
N2(g) + 3H2(g) 2NH3(g)
A) 0.173 M/s
B) 0.345 M/s
C) 0.690 M/s
D) 245 M/s
E) 0.518 M/s

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
35
If the rate of the reaction:
2O3(g) 3O2(g)
Is 0.250 M/s over the first 5.50 s, how much oxygen will form during this time?
A) 1.38 M
B) 4.13 M
C) 0.69 M
D) 0.25 M
E) 0.46 M
2O3(g) 3O2(g)
Is 0.250 M/s over the first 5.50 s, how much oxygen will form during this time?
A) 1.38 M
B) 4.13 M
C) 0.69 M
D) 0.25 M
E) 0.46 M

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
36
Which of the following is not a possible graph of concentration versus time for a reactant?
A)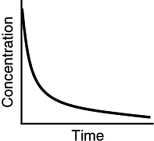
B)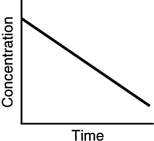
C)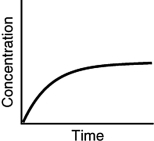
D)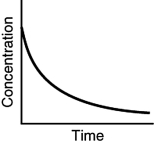
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
37
In the combustion of methane, CH4(g) + 2 O2(g) CO2(g) + 2 H2O (g), which reactant has the greatest rate of disappearance?
A) CH4
B) O2
C) CO2
D) H2O
E) CH4 and O2 have the same rate of disappearance.
A) CH4
B) O2
C) CO2
D) H2O
E) CH4 and O2 have the same rate of disappearance.

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
38
Assuming that each of the following graphs has the same concentration and time axes, which has the greatest initial rate of disappearance of reactant?
A)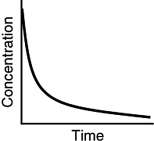
B)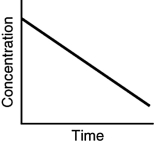
C)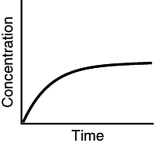
D)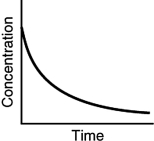
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
39
One reaction that occurs in an automobile catalytic converter is the conversion of carbon monoxide to carbon dioxide. How is the rate of this reaction related to the rate at which the concentration of a reactant or product changes?
2CO(g) + O2(g) 2CO2(g)
I. Rate = [CO]/ t
II. Rate = - [CO]/ t
III. Rate = - [O2]/ t
IV. Rate = [CO2]/ t
A) I only
B) II only
C) III only
D) II and III only
E) II, III, and IV only
2CO(g) + O2(g) 2CO2(g)
I. Rate = [CO]/ t
II. Rate = - [CO]/ t
III. Rate = - [O2]/ t
IV. Rate = [CO2]/ t
A) I only
B) II only
C) III only
D) II and III only
E) II, III, and IV only

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
40
If the rate of formation of ammonia is 0.345 M/s, what is the rate of disappearance of N2?
A) 0.173 M/s
B) 0.345 M/s
C) 0.690 M/s
D) 245 M/s
E) 0.518 M/s
A) 0.173 M/s
B) 0.345 M/s
C) 0.690 M/s
D) 245 M/s
E) 0.518 M/s

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
41
Which of these could be the units of the rate constant for a first-order reaction?
A) M/s
B) 1/Ms
C) 1/Ms2
D) 1/s
E) Ms
A) M/s
B) 1/Ms
C) 1/Ms2
D) 1/s
E) Ms

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
42
Which of these could be the units for the rate constant in a second-order reaction?
A) M-1 s
B) M s-1
C) M-1 s-1
D) M-2 s-1
E) Ms
A) M-1 s
B) M s-1
C) M-1 s-1
D) M-2 s-1
E) Ms

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
43
A reaction is first order in A. If the rate constant of the reaction is 3.45 *10-3 s-1, what is the half-life (t1/2) of the reaction?
A) 4.98 *10-3 s
B) 200 s
C) 3.45 *10-3 s
D) 100 s
E) 1.73 *10-3 s
A) 4.98 *10-3 s
B) 200 s
C) 3.45 *10-3 s
D) 100 s
E) 1.73 *10-3 s

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
44
Given the following data, determine the order of the reaction with respect to Cl2.
2NO(g) + Cl2(g) 2NOCl(g)
A) first
B) second
C) third
D) fourth
E) fifth
2NO(g) + Cl2(g) 2NOCl(g)
A) first
B) second
C) third
D) fourth
E) fifth

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
45
If the reaction 3A + B + C 2D + E is first order overall, which of these could be the units of its rate constant, k?
A) 1/s
B) M/s
C) 1/Ms
D) 1/Ms2
E) Ms
A) 1/s
B) M/s
C) 1/Ms
D) 1/Ms2
E) Ms

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
46
For the rate law Rate = k[A]3/2[B], the partial order with respect to A is ________, the partial order with respect to B is ________, and the total order is ________.
A)
B)
C)
D)
E) The orders cannot be determined without a chemical reaction.
A)
B)
C)
D)
E) The orders cannot be determined without a chemical reaction.

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
47
The reaction 2NO(g) + O2(g) 2NO2(g) has the following rate law: Rate = k[O2][NO]2. If the concentration of NO is reduced by a factor of two, the rate will ________
A) double.
B) quadruple.
C) be reduced by one-quarter.
D) be reduced by one-half.
E) remain the same.
A) double.
B) quadruple.
C) be reduced by one-quarter.
D) be reduced by one-half.
E) remain the same.

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
48
Given the following data, determine the order of the reaction with respect to NO(g).
2NO(g) + Cl2(g) 2NOCl(g)
A) first
B) second
C) third
D) fourth
E) fifth
2NO(g) + Cl2(g) 2NOCl(g)
A) first
B) second
C) third
D) fourth
E) fifth

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
49
The second-order reaction A B is found to have a rate constant of 0.978 M-1s-1. What is the half-life of this reaction when [A]0 = 0.0300 M?
A) 0.0293 s
B) 0.710 s
C) 34.1 s
D) 60.1 s
E) 24.3 s
A) 0.0293 s
B) 0.710 s
C) 34.1 s
D) 60.1 s
E) 24.3 s

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
50
A second-order reaction (2A B) with a rate constant of 0.350 M-1s-1 is found to have a half-life of 3.45 s. What was the initial concentration of the reactant, [A]0?
A) 0.828 M
B) 0.201 M
C) 1.00 M
D) 1.21 M
E) 0.350 M
A) 0.828 M
B) 0.201 M
C) 1.00 M
D) 1.21 M
E) 0.350 M

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
51
For the rate law Rate = k[A]1/2[B], the partial order with respect to A is ________, the partial order with respect to B is ________, and the total order is ________.
A)
B)
C)
D) .
E) The orders cannot be determined without a chemical reaction.
A)
B)
C)
D) .
E) The orders cannot be determined without a chemical reaction.

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
52
Given the following data, determine the order of the reaction with respect to H2.
H2(g) + 2ICl(g) I2(g) + 2HCl(g)
A) one-half
B) second
C) first
D) third
E) three-halves
H2(g) + 2ICl(g) I2(g) + 2HCl(g)
A) one-half
B) second
C) first
D) third
E) three-halves

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
53
The half-life (t1/2) of a first-order reaction is 0.100 s. What is the rate constant?
A) 6.93 s-1
B) 0.693 s-1
C) 0.0693 s-1
D) 0.144 s-1
E) 3.01 s-1
A) 6.93 s-1
B) 0.693 s-1
C) 0.0693 s-1
D) 0.144 s-1
E) 3.01 s-1

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
54
For the rate law Rate = k[A][B]3/2, the partial order with respect to A is ________, the partial order with respect to B is ________, and the total order is ________.
A)
B)
C)
D)
E) The orders cannot be determined without a chemical reaction.
A)
B)
C)
D)
E) The orders cannot be determined without a chemical reaction.

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
55
For the rate law Rate = k[A][B]1/2, the partial order with respect to A is ________, the partial order with respect to B is ________, and the total order is ________.
A)
B)
C)
D)
E) The orders cannot be determined without a chemical reaction.
A)
B)
C)
D)
E) The orders cannot be determined without a chemical reaction.

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
56
The reaction
A + 2B C
Is first order in B and A. The overall order of the reaction is ________
A) first.
B) second.
C) third.
D) zero.
E) fourth.
A + 2B C
Is first order in B and A. The overall order of the reaction is ________
A) first.
B) second.
C) third.
D) zero.
E) fourth.

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
57
Given the following data, determine the order of the reaction with respect to ICl.
H2(g) + 2ICl(g) I2(g) + 2HCl(g)
A) one-half
B) first
C) second
D) third
E) three-halves
H2(g) + 2ICl(g) I2(g) + 2HCl(g)
A) one-half
B) first
C) second
D) third
E) three-halves

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
58
The reaction CHCl3(g) + Cl2(g) CCl4(g) + HCl(g) has the following rate law:
Rate = k[CHCl3][Cl2]. If the concentration of CHCl3 is increased by a factor of five while the concentration of Cl2 is kept the same, the rate will ________
A) double.
B) triple.
C) stay the same.
D) increase by a factor of five.
E) decrease by a factor of one-fifth.
Rate = k[CHCl3][Cl2]. If the concentration of CHCl3 is increased by a factor of five while the concentration of Cl2 is kept the same, the rate will ________
A) double.
B) triple.
C) stay the same.
D) increase by a factor of five.
E) decrease by a factor of one-fifth.

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
59
Given the following data, determine the rate law for the reaction
NH4+(aq) + NO2-(aq) N2(g) + 2H2O(l)
A) k[NH4+][NO2-]
B) k[NH4+]2[NO2-]
C) k[NH4+][NO2-]1/2
D) k[NH4+]1/2[NO2-]2
E) k[NH4+][NO2-]2
NH4+(aq) + NO2-(aq) N2(g) + 2H2O(l)
A) k[NH4+][NO2-]
B) k[NH4+]2[NO2-]
C) k[NH4+][NO2-]1/2
D) k[NH4+]1/2[NO2-]2
E) k[NH4+][NO2-]2

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
60
In a rate law, the partial orders are determined by ________
A) the reactant concentrations.
B) the stoichiometric coefficients.
C) the product concentrations.
D) experiment.
E) the difference between the forward and reverse rates.
A) the reactant concentrations.
B) the stoichiometric coefficients.
C) the product concentrations.
D) experiment.
E) the difference between the forward and reverse rates.

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
61
The initial rate data for the reaction 2N2O5(g) 4NO2(g) + O2(g) is shown in the following table. Determine the value of the rate constant for this reaction.
A) 4.09 s-1
B) 0.176 s-1
C) 0.0569 s-1
D) 0.225 s-1
E) 80.1 s-1
A) 4.09 s-1
B) 0.176 s-1
C) 0.0569 s-1
D) 0.225 s-1
E) 80.1 s-1

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
62
Determine the overall order of the reaction H2(g) + 2ICl(g) I2(g) + 2HCl(g) from the following data:
A) first
B) second
C) third
D) fourth
E) zero
A) first
B) second
C) third
D) fourth
E) zero

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
63
Determine the order of the reaction CH3CHO (g) CH4(g) + CO(g) from the following data:
A) one-half
B) first
C) second
D) three-halves
E) third
A) one-half
B) first
C) second
D) three-halves
E) third

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
64
Indicate which of the following plots would be obtained for a second-order reaction.
A)
B)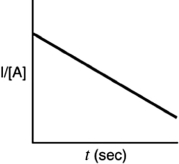
C)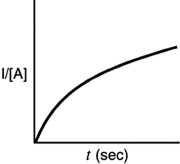
D)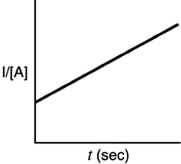
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
65
Given the following data, determine the rate law for the reaction
H2(g) + 2ICl(g) I2(g) + 2HCl(g)
A) Rate = k[H2][ICl]
B) Rate = k[H2][ICl]2
C) Rate = k[H2]2[ICl]
D) Rate = k[H2]1/2[ICl]1/2
E) Rate = k[H2][ICl]1/2
H2(g) + 2ICl(g) I2(g) + 2HCl(g)
A) Rate = k[H2][ICl]
B) Rate = k[H2][ICl]2
C) Rate = k[H2]2[ICl]
D) Rate = k[H2]1/2[ICl]1/2
E) Rate = k[H2][ICl]1/2

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
66
Chlorine dioxide (ClO2) is used as a disinfectant in municipal water-treatment plants. It decomposes in a first-order reaction with a rate constant of 14 s-1. How long would it take for an initial concentration of 0.06 M to decrease to 0.02 M?
A) 13 ms
B) 78 ms
C) 92 ms
D) 146 ms
E) 239 ms
A) 13 ms
B) 78 ms
C) 92 ms
D) 146 ms
E) 239 ms

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
67
Chlorine dioxide (ClO2) is used as a disinfectant in municipal water-treatment plants. It decomposes in a first-order reaction with a rate constant of 0.0714 s-1. If the initial concentration were 0.12 M, what would the concentration be after 14.0 s has elapsed?
A) 0.033 M
B) 0.12 M
C) 0.060 M
D) 0.012 M
E) 0.044 M
A) 0.033 M
B) 0.12 M
C) 0.060 M
D) 0.012 M
E) 0.044 M

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
68
Given the following data, determine the rate constant of the reaction
2NO(g) + Cl2(g) 2NOCl(g)
A) 1.13 M-2 s-1
B) 9.44 M-2 s-1
C) 37.8 M-2 s-1
D) 0.0265 M-2 s-1
E) 59.6 M-2 s-1
2NO(g) + Cl2(g) 2NOCl(g)
A) 1.13 M-2 s-1
B) 9.44 M-2 s-1
C) 37.8 M-2 s-1
D) 0.0265 M-2 s-1
E) 59.6 M-2 s-1

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
69
The second-order reaction A + B C will obey pseudo-first-order kinetics when ________
A) the concentrations of A and B are both small.
B) the concentration of one of the reactants does not change.
C) the concentration of C is large.
D) the concentrations of A and B are both large.
E) a catalyst changes the mechanism to a first-order reaction.
A) the concentrations of A and B are both small.
B) the concentration of one of the reactants does not change.
C) the concentration of C is large.
D) the concentrations of A and B are both large.
E) a catalyst changes the mechanism to a first-order reaction.

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
70
Determine the overall order of the reaction 2NO(g) + Cl2(g) 2NOCl(g) from the following data:
A) first
B) second
C) third
D) fourth
E) fifth
A) first
B) second
C) third
D) fourth
E) fifth

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
71
Given the following data, determine the rate law for the reaction
2NO(g) + Cl2(g) 2NOCl(g)
A) Rate = k[NO][Cl2]
B) Rate = k[NO][Cl2]2
C) Rate = k[NO]2[Cl2]
D) Rate = k[NO]2[Cl2]2
E) Rate = k[NO][Cl2]1/2
2NO(g) + Cl2(g) 2NOCl(g)
A) Rate = k[NO][Cl2]
B) Rate = k[NO][Cl2]2
C) Rate = k[NO]2[Cl2]
D) Rate = k[NO]2[Cl2]2
E) Rate = k[NO][Cl2]1/2

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
72
The first-order reaction A B, has k = 5.67 s-1. If [A]0 = 0.500 M, how long will it take to reach [A] = 0.124 M?
A) 0.122 s
B) 0.100 s
C) 8.18 s
D) 0.246 s
E) 0.488 s
A) 0.122 s
B) 0.100 s
C) 8.18 s
D) 0.246 s
E) 0.488 s

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
73
Which of the following plots indicates that the reaction is zero order?
A)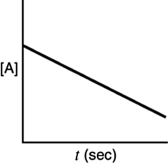
B)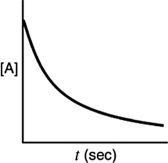
C)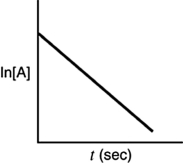
D)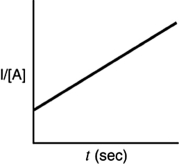
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
74
The linear form of ________ is very useful, as it allows us to calculate the activation energy and the frequency factor.
A) the Boltzmann equation
B) the Arrhenius equation
C) Planck's equation
D) the rate law
E) the integrated rate law
A) the Boltzmann equation
B) the Arrhenius equation
C) Planck's equation
D) the rate law
E) the integrated rate law

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
75
Given the following data, determine the rate constant, k, of the reaction
H2(g) + 2ICl(g) I2(g) + 2HCl(g)
A) 1.65 *10-5 torr -1s-1
B) 6.06 *104 torr -1s-1
C) 8.17 *10-5 torr -1s-1
D) 1.34 torr -1s-1
E) 3.48 *103 torr -1s-1
H2(g) + 2ICl(g) I2(g) + 2HCl(g)
A) 1.65 *10-5 torr -1s-1
B) 6.06 *104 torr -1s-1
C) 8.17 *10-5 torr -1s-1
D) 1.34 torr -1s-1
E) 3.48 *103 torr -1s-1

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
76
Which of the following plots would indicate that a reaction was first order?
A)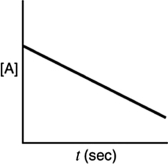
B)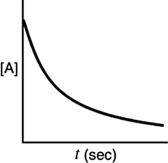
C)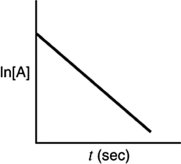
D)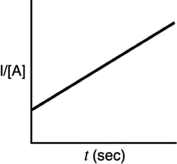
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
77
Given the following data, determine the rate law for the reaction
CH3CHO(g) CH4(g) + CO(g)
A) Rate = k
B) Rate = kP[CH3CHO]
C) Rate = kP[CH3CHO]3/2
D) Rate = kP[CH3CHO]1/2
E) Rate = kP[CH3CHO]2
CH3CHO(g) CH4(g) + CO(g)
A) Rate = k
B) Rate = kP[CH3CHO]
C) Rate = kP[CH3CHO]3/2
D) Rate = kP[CH3CHO]1/2
E) Rate = kP[CH3CHO]2

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
78
Collision theory assumes that the rate of a reaction depends on ________
A) the energy of collisions.
B) the orientation of colliding molecules.
C) the energy of collisions and the orientation of colliding molecules.
D) the change in energy between the products and the reactants.
E) the change in free energy between the reactants and products.
A) the energy of collisions.
B) the orientation of colliding molecules.
C) the energy of collisions and the orientation of colliding molecules.
D) the change in energy between the products and the reactants.
E) the change in free energy between the reactants and products.

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
79
Which of the following plots indicates that the reaction is second order?
A)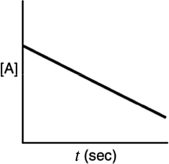
B)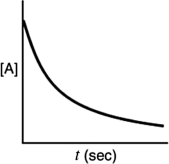
C)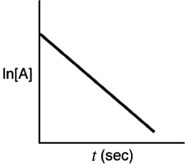
D)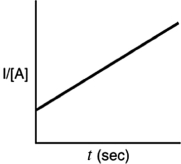
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
80
Indicate which of the following plots would be obtained for a first-order reaction.
A)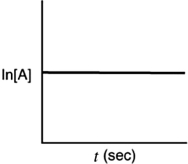
B)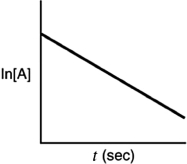
C)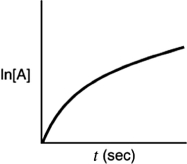
D)
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck



