Deck 10: The Shapes of Molecules
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/108
Play
Full screen (f)
Deck 10: The Shapes of Molecules
1
Oxygen difluoride is a powerful oxidizing and fluorinating agent. Select its Lewis structure.
A)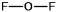
B)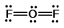
C)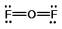
D)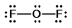
E) None of these choices is correct.
A)
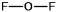
B)
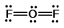
C)
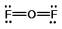
D)
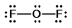
E) None of these choices is correct.
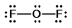
2
In which one of the following is the best Lewis structure a resonance structure?
A) SO3
B) BF3
C) I3¯
D) SCO (C = central atom)
E) SO32¯
A) SO3
B) BF3
C) I3¯
D) SCO (C = central atom)
E) SO32¯
SO32¯
3
In which one of the following species is the best Lewis structure a resonance structure?
A) NH3
B) CO2
C) SF6
D) O2
E) CO32¯
A) NH3
B) CO2
C) SF6
D) O2
E) CO32¯
CO32¯
4
In which one of the following molecules are all the bonds single?
A) O3
B) POCl3
C) CO
D) COCl2
E) N2H4
A) O3
B) POCl3
C) CO
D) COCl2
E) N2H4

Unlock Deck
Unlock for access to all 108 flashcards in this deck.
Unlock Deck
k this deck
5
Which one of the following molecules contains a double bond?
A) N2
B) PCl5
C) CH2O
D) C2H2
E) I2
A) N2
B) PCl5
C) CH2O
D) C2H2
E) I2

Unlock Deck
Unlock for access to all 108 flashcards in this deck.
Unlock Deck
k this deck
6
Select the best Lewis structure for ClCN.
A)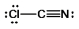
B)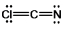
C)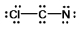
D)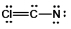
E)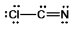
A)
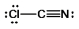
B)
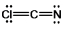
C)
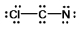
D)
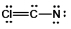
E)
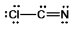

Unlock Deck
Unlock for access to all 108 flashcards in this deck.
Unlock Deck
k this deck
7
Select the correct Lewis structure for TeBr2.
A)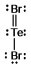
B)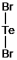
C)
D)
E)
A)
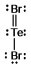
B)
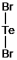
C)

D)

E)


Unlock Deck
Unlock for access to all 108 flashcards in this deck.
Unlock Deck
k this deck
8
In which one of the following is the best Lewis structure a resonance structure?
A) CO2 (C = central atom)
B) ClO3¯ (Cl = central atom)
C) COCl2 (C = central atom)
D) NO2+ (N = central atom)
E) HCN (C = central atom)
A) CO2 (C = central atom)
B) ClO3¯ (Cl = central atom)
C) COCl2 (C = central atom)
D) NO2+ (N = central atom)
E) HCN (C = central atom)

Unlock Deck
Unlock for access to all 108 flashcards in this deck.
Unlock Deck
k this deck
9
In carbon disulfide, how many lone pairs of electrons are on each sulfur atom?
A) 0
B) 1
C) 2
D) 3
E) 4
A) 0
B) 1
C) 2
D) 3
E) 4

Unlock Deck
Unlock for access to all 108 flashcards in this deck.
Unlock Deck
k this deck
10
Hydrazine, N2H4, is a good reducing agent that has been used as a component in rocket fuels. Select its Lewis structure.
A)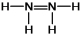
B)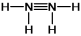
C)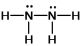
D)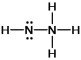
E) None of these choices is correct.
A)
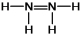
B)
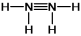
C)

D)

E) None of these choices is correct.

Unlock Deck
Unlock for access to all 108 flashcards in this deck.
Unlock Deck
k this deck
11
Select the best Lewis structure for P2I4.
A)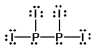
B)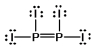
C)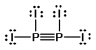
D)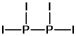
E) None of these structures is suitable for P2I4.
A)

B)
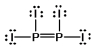
C)
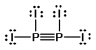
D)
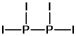
E) None of these structures is suitable for P2I4.

Unlock Deck
Unlock for access to all 108 flashcards in this deck.
Unlock Deck
k this deck
12
How many electron pairs are shared between the carbon atoms in C2H4?
A) 5
B) 4
C) 3
D) 2
E) 1
A) 5
B) 4
C) 3
D) 2
E) 1

Unlock Deck
Unlock for access to all 108 flashcards in this deck.
Unlock Deck
k this deck
13
Select the correct Lewis structure for nitrogen trifluoride, NF3.
A)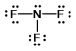
B)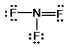
C)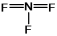
D)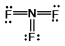
E)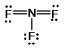
A)

B)
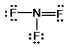
C)
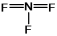
D)
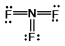
E)
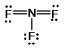

Unlock Deck
Unlock for access to all 108 flashcards in this deck.
Unlock Deck
k this deck
14
In the nitrate ion (NO3¯), nitrogen and oxygen are held together by
A) ionic interactions.
B) covalent bonds.
C) dative bonds.
D) electronegativity.
E) network bonds.
A) ionic interactions.
B) covalent bonds.
C) dative bonds.
D) electronegativity.
E) network bonds.

Unlock Deck
Unlock for access to all 108 flashcards in this deck.
Unlock Deck
k this deck
15
How many resonance structures are possible for NO3¯?
A) 1
B) 2
C) 3
D) 4
E) 5
A) 1
B) 2
C) 3
D) 4
E) 5

Unlock Deck
Unlock for access to all 108 flashcards in this deck.
Unlock Deck
k this deck
16
Which one of the following Lewis structures is definitely incorrect?
A) NO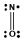
B) HCN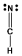
C) NO2¯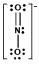
D) SO32¯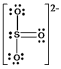
E) PCl5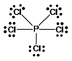
A) NO
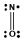
B) HCN
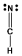
C) NO2¯

D) SO32¯

E) PCl5


Unlock Deck
Unlock for access to all 108 flashcards in this deck.
Unlock Deck
k this deck
17
Thionyl chloride is used as an oxidizing and chlorinating agent in organic chemistry. Select the best Lewis structure for SOCl2.
A)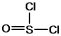
B)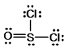
C)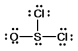
D)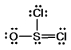
E) None of these structures is suitable for SOCl2.
A)
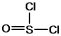
B)
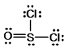
C)

D)
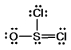
E) None of these structures is suitable for SOCl2.

Unlock Deck
Unlock for access to all 108 flashcards in this deck.
Unlock Deck
k this deck
18
In neutral molecules, how many bonds are commonly formed by nitrogen? And how many by oxygen?
A) 5 and 6
B) 4 and 2
C) 3 and 6
D) 4 and 6
E) 3 and 2
A) 5 and 6
B) 4 and 2
C) 3 and 6
D) 4 and 6
E) 3 and 2

Unlock Deck
Unlock for access to all 108 flashcards in this deck.
Unlock Deck
k this deck
19
Select the correct Lewis structure for NOCl, a reactive material used as an ionizing solvent.
A)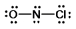
B)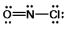
C)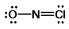
D)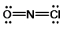
E) None of these choices is correct.
A)
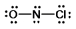
B)
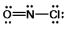
C)
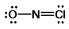
D)
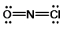
E) None of these choices is correct.

Unlock Deck
Unlock for access to all 108 flashcards in this deck.
Unlock Deck
k this deck
20
Which one of the following Lewis structures is definitely incorrect?
A) NO2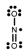
B) BeCl2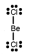
C) CO32¯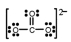
D) CH4
E) SO2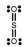
A) NO2
B) BeCl2
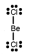
C) CO32¯

D) CH4

E) SO2
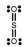

Unlock Deck
Unlock for access to all 108 flashcards in this deck.
Unlock Deck
k this deck
21
In which one of the following structures does the central atom have a formal charge of +2?
A) SF6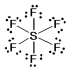
B) SO42¯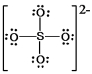
C) O3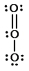
D) BeCl2
E) AlCl4¯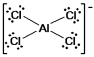
A) SF6

B) SO42¯

C) O3

D) BeCl2

E) AlCl4¯


Unlock Deck
Unlock for access to all 108 flashcards in this deck.
Unlock Deck
k this deck
22
Which of the following atoms can expand its valence shell when bonding?
A) N
B) C
C) O
D) P
E) Al
A) N
B) C
C) O
D) P
E) Al

Unlock Deck
Unlock for access to all 108 flashcards in this deck.
Unlock Deck
k this deck
23
How many faces and how many vertexes (corners), respectively, are there in a trigonal bipyramid?
A) 4 and 4
B) 5 and 5
C) 5 and 6
D) 6 and 5
E) 6 and 8
A) 4 and 4
B) 5 and 5
C) 5 and 6
D) 6 and 5
E) 6 and 8

Unlock Deck
Unlock for access to all 108 flashcards in this deck.
Unlock Deck
k this deck
24
According to VSEPR theory, a molecule with the general formula AX3 will have a ______ molecular shape.
A) linear
B) bent
C) trigonal planar
D) tetrahedral
E) trigonal pyramidal
A) linear
B) bent
C) trigonal planar
D) tetrahedral
E) trigonal pyramidal

Unlock Deck
Unlock for access to all 108 flashcards in this deck.
Unlock Deck
k this deck
25
Phosphoryl iodide is used in the preparation of organophosphorus derivatives and phosphate esters. Select the Lewis structure for POI3 which minimizes formal charges.
A)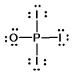
B)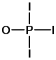
C)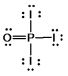
D)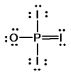
E)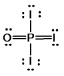
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 108 flashcards in this deck.
Unlock Deck
k this deck
26
Considering all the bonds in a molecule with trigonal bipyramidal geometry, what are the bond angles present?
A) 120 only
B) 90 only
C) 180 only
D) 60 and 90 only
E) 90 , 120 and 180
A) 120 only
B) 90 only
C) 180 only
D) 60 and 90 only
E) 90 , 120 and 180

Unlock Deck
Unlock for access to all 108 flashcards in this deck.
Unlock Deck
k this deck
27
In which of the following does the nitrogen atom have a formal charge of -1?
A)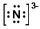
B)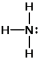
C)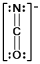
D)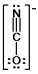
E)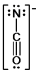
A)
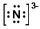
B)
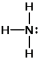
C)

D)

E)


Unlock Deck
Unlock for access to all 108 flashcards in this deck.
Unlock Deck
k this deck
28
In which one of the following species is the central atom (the first atom in the formula) likely to violate the octet rule?
A) BF4¯
B) XeO3
C) SiCl4
D) NH3
E) CH2Cl2
A) BF4¯
B) XeO3
C) SiCl4
D) NH3
E) CH2Cl2

Unlock Deck
Unlock for access to all 108 flashcards in this deck.
Unlock Deck
k this deck
29
Select the Lewis structure in which formal charges are minimized for the periodate anion, IO4¯.
A)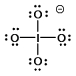
B)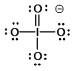
C)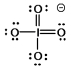
D)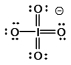
E)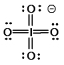
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 108 flashcards in this deck.
Unlock Deck
k this deck
30
The formal charges on Cl and O in the structure shown for the ClO¯ ion are, respectively: 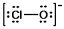
A) 0 and -1
B) -1 and 0
C) 1 and -2
D) -2 and 1
E) None of these choices is correct.
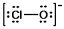
A) 0 and -1
B) -1 and 0
C) 1 and -2
D) -2 and 1
E) None of these choices is correct.

Unlock Deck
Unlock for access to all 108 flashcards in this deck.
Unlock Deck
k this deck
31
According to VSEPR theory, a molecule with the general formula AX4 will have a ______ molecular shape.
A) bent
B) trigonal planar
C) trigonal pyramidal
D) square planar
E) tetrahedral
A) bent
B) trigonal planar
C) trigonal pyramidal
D) square planar
E) tetrahedral

Unlock Deck
Unlock for access to all 108 flashcards in this deck.
Unlock Deck
k this deck
32
The best Lewis structure for sulfuric acid has zero formal charges, sulfur as the central atom, and no bonds between S and H. How many single and double bonds, respectively, are there in this Lewis structure?
A) 2 single, 4 double
B) 4 single, 2 double
C) 4 single, no double
D) 6 single, no double
E) 5 single, 1 double
A) 2 single, 4 double
B) 4 single, 2 double
C) 4 single, no double
D) 6 single, no double
E) 5 single, 1 double

Unlock Deck
Unlock for access to all 108 flashcards in this deck.
Unlock Deck
k this deck
33
In the COCl2 molecule, carbon is the central atom. Based on the best Lewis structure for COCl2, what is the formal charge on carbon?
A) 0
B) +1
C) -1
D) +2
E) -2
A) 0
B) +1
C) -1
D) +2
E) -2

Unlock Deck
Unlock for access to all 108 flashcards in this deck.
Unlock Deck
k this deck
34
According to VSEPR theory, a molecule with the general formula AX2 will have a ___ molecular shape.
A) linear
B) bent
C) trigonal planar
D) tetrahedral
E) triangular
A) linear
B) bent
C) trigonal planar
D) tetrahedral
E) triangular

Unlock Deck
Unlock for access to all 108 flashcards in this deck.
Unlock Deck
k this deck
35
In which one of the following species is the central atom (the first atom in the formula) an exception to the octet rule?
A) NH3
B) NH4+
C) I2
D) BH4¯
E) SF6
A) NH3
B) NH4+
C) I2
D) BH4¯
E) SF6

Unlock Deck
Unlock for access to all 108 flashcards in this deck.
Unlock Deck
k this deck
36
In the following Lewis structure for phosphate, phosphorus has a formal charge of ____ and an oxidation number of ____. 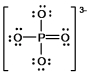
A) 0, -3
B) 0, 5
C) 5, -3
D) 5, 5
E) 3, 5

A) 0, -3
B) 0, 5
C) 5, -3
D) 5, 5
E) 3, 5

Unlock Deck
Unlock for access to all 108 flashcards in this deck.
Unlock Deck
k this deck
37
The formal charge on Cl in the structure shown for the perchlorate ion is: 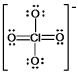
A) -2
B) -1
C) 0
D) +1
E) +2

A) -2
B) -1
C) 0
D) +1
E) +2

Unlock Deck
Unlock for access to all 108 flashcards in this deck.
Unlock Deck
k this deck
38
Select the Lewis structure for XeO2F2 which correctly minimizes formal charges.
A)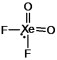
B)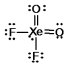
C)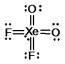
D)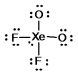
E)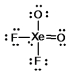
A)

B)
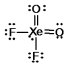
C)
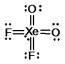
D)

E)
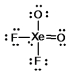

Unlock Deck
Unlock for access to all 108 flashcards in this deck.
Unlock Deck
k this deck
39
According to VSEPR theory, a molecule with the general formula AX5 will have a ______ molecular shape.
A) tetrahedral
B) trigonal planar
C) trigonal pyramidal
D) trigonal bipyramidal
E) see-saw
A) tetrahedral
B) trigonal planar
C) trigonal pyramidal
D) trigonal bipyramidal
E) see-saw

Unlock Deck
Unlock for access to all 108 flashcards in this deck.
Unlock Deck
k this deck
40
In the following Lewis structure for ClO3F, chlorine has a formal charge of ____ and an oxidation number of ____. 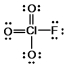
A) 7, 7
B) 7, -1
C) 1, 1
D) 1, -1
E) 1, 7

A) 7, 7
B) 7, -1
C) 1, 1
D) 1, -1
E) 1, 7

Unlock Deck
Unlock for access to all 108 flashcards in this deck.
Unlock Deck
k this deck
41
What is the molecular shape of NO2¯ as predicted by the VSEPR theory?
A) linear
B) trigonal planar
C) bent
D) tetrahedral
E) resonant
A) linear
B) trigonal planar
C) bent
D) tetrahedral
E) resonant

Unlock Deck
Unlock for access to all 108 flashcards in this deck.
Unlock Deck
k this deck
42
What is the molecular shape of N2O as predicted by the VSEPR theory? 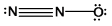
A) trigonal pyramidal
B) trigonal planar
C) angular
D) bent
E) linear
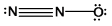
A) trigonal pyramidal
B) trigonal planar
C) angular
D) bent
E) linear

Unlock Deck
Unlock for access to all 108 flashcards in this deck.
Unlock Deck
k this deck
43
What is the molecular shape of NH2Cl as predicted by the VSEPR theory?
A) trigonal pyramidal
B) tetrahedral
C) T-shaped
D) see-saw
E) trigonal planar
A) trigonal pyramidal
B) tetrahedral
C) T-shaped
D) see-saw
E) trigonal planar

Unlock Deck
Unlock for access to all 108 flashcards in this deck.
Unlock Deck
k this deck
44
What is the molecular shape of the thiocyanate anion, SCN¯, as predicted by the VSEPR theory? (Carbon is the central atom.)
A) linear
B) bent
C) angular
D) trigonal
E) None of these choices is correct.
A) linear
B) bent
C) angular
D) trigonal
E) None of these choices is correct.

Unlock Deck
Unlock for access to all 108 flashcards in this deck.
Unlock Deck
k this deck
45
What is the molecular shape of NOCl as predicted by the VSEPR theory? 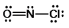
A) linear
B) trigonal planar
C) bent
D) tetrahedral
E) trigonal pyramidal
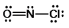
A) linear
B) trigonal planar
C) bent
D) tetrahedral
E) trigonal pyramidal

Unlock Deck
Unlock for access to all 108 flashcards in this deck.
Unlock Deck
k this deck
46
According to VSEPR theory, a molecule with the general formula AX2E3 will have a _____ molecular shape.
A) bent
B) linear
C) trigonal planar
D) T-shaped
E) trigonal pyramidal
A) bent
B) linear
C) trigonal planar
D) T-shaped
E) trigonal pyramidal

Unlock Deck
Unlock for access to all 108 flashcards in this deck.
Unlock Deck
k this deck
47
What is the molecular shape of ClCN as predicted by the VSEPR theory? (Carbon is the central atom.)
A) linear
B) bent
C) angular
D) trigonal
E) None of these choices is correct.
A) linear
B) bent
C) angular
D) trigonal
E) None of these choices is correct.

Unlock Deck
Unlock for access to all 108 flashcards in this deck.
Unlock Deck
k this deck
48
According to VSEPR theory, a molecule with the general formula AX3E2 will have a _____ molecular shape.
A) trigonal pyramidal
B) trigonal bipyramidal
C) trigonal planar
D) T-shaped
E) see-saw
A) trigonal pyramidal
B) trigonal bipyramidal
C) trigonal planar
D) T-shaped
E) see-saw

Unlock Deck
Unlock for access to all 108 flashcards in this deck.
Unlock Deck
k this deck
49
What is the molecular shape of BeH2 as predicted by the VSEPR theory?
A) linear
B) bent
C) angular
D) trigonal
E) None of these choices is correct.
A) linear
B) bent
C) angular
D) trigonal
E) None of these choices is correct.

Unlock Deck
Unlock for access to all 108 flashcards in this deck.
Unlock Deck
k this deck
50
According to VSEPR theory, a molecule with the general formula AX2E2 will have a _____ molecular shape.
A) linear
B) bent
C) trigonal planar
D) tetrahedral
E) see-saw
A) linear
B) bent
C) trigonal planar
D) tetrahedral
E) see-saw

Unlock Deck
Unlock for access to all 108 flashcards in this deck.
Unlock Deck
k this deck
51
According to VSEPR theory, a molecule with the general formula AX5E will have a ______ molecular shape.
A) tetrahedral
B) trigonal bipyramidal
C) square pyramidal
D) octahedral
E) see-saw
A) tetrahedral
B) trigonal bipyramidal
C) square pyramidal
D) octahedral
E) see-saw

Unlock Deck
Unlock for access to all 108 flashcards in this deck.
Unlock Deck
k this deck
52
What is the molecular shape of HOF as predicted by the VSEPR theory?
A) trigonal pyramidal
B) trigonal
C) tetrahedral
D) linear
E) bent
A) trigonal pyramidal
B) trigonal
C) tetrahedral
D) linear
E) bent

Unlock Deck
Unlock for access to all 108 flashcards in this deck.
Unlock Deck
k this deck
53
What is the molecular shape of ClO3F as predicted by the VSEPR theory? 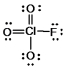
A) trigonal pyramidal
B) square planar
C) square pyramidal
D) tetrahedral
E) octahedral

A) trigonal pyramidal
B) square planar
C) square pyramidal
D) tetrahedral
E) octahedral

Unlock Deck
Unlock for access to all 108 flashcards in this deck.
Unlock Deck
k this deck
54
What is the molecular shape of BCl3 as predicted by the VSEPR theory?
A) linear
B) trigonal planar
C) bent
D) tetrahedral
E) trigonal pyramidal
A) linear
B) trigonal planar
C) bent
D) tetrahedral
E) trigonal pyramidal

Unlock Deck
Unlock for access to all 108 flashcards in this deck.
Unlock Deck
k this deck
55
According to VSEPR theory, a molecule with the general formula AX3E will have a _____ molecular shape.
A) bent
B) trigonal planar
C) trigonal pyramidal
D) tetrahedral
E) triangular
A) bent
B) trigonal planar
C) trigonal pyramidal
D) tetrahedral
E) triangular

Unlock Deck
Unlock for access to all 108 flashcards in this deck.
Unlock Deck
k this deck
56
What is the molecular symmetry around the carbons in CCl2CH2 as predicted by the VSEPR theory?
A) linear
B) trigonal planar
C) V-shaped
D) tetrahedral
E) trigonal pyramidal
A) linear
B) trigonal planar
C) V-shaped
D) tetrahedral
E) trigonal pyramidal

Unlock Deck
Unlock for access to all 108 flashcards in this deck.
Unlock Deck
k this deck
57
According to VSEPR theory, a molecule with the general formula AX4E2 will have a _____ molecular shape.
A) tetrahedral
B) square pyramidal
C) square planar
D) octahedral
E) see-saw
A) tetrahedral
B) square pyramidal
C) square planar
D) octahedral
E) see-saw

Unlock Deck
Unlock for access to all 108 flashcards in this deck.
Unlock Deck
k this deck
58
According to VSEPR theory, a molecule with the general formula AX6 will have a ______ molecular shape.
A) tetrahedral
B) trigonal planar
C) trigonal bipyramidal
D) hexagonal
E) octahedral
A) tetrahedral
B) trigonal planar
C) trigonal bipyramidal
D) hexagonal
E) octahedral

Unlock Deck
Unlock for access to all 108 flashcards in this deck.
Unlock Deck
k this deck
59
According to VSEPR theory, a molecule with the general formula AX2E will have a ______ molecular shape.
A) bent
B) see-saw
C) trigonal planar
D) T-shaped
E) trigonal pyramidal
A) bent
B) see-saw
C) trigonal planar
D) T-shaped
E) trigonal pyramidal

Unlock Deck
Unlock for access to all 108 flashcards in this deck.
Unlock Deck
k this deck
60
According to VSEPR theory, a molecule with the general formula AX4E will have a _____ molecular shape.
A) bent
B) see-saw
C) trigonal planar
D) T-shaped
E) square planar
A) bent
B) see-saw
C) trigonal planar
D) T-shaped
E) square planar

Unlock Deck
Unlock for access to all 108 flashcards in this deck.
Unlock Deck
k this deck
61
Predict the ideal bond angles around nitrogen in N2F2 using the molecular shape given by the VSEPR theory. (The two N atoms are the central atoms.)
A) 90
B) 109
C) 120
D) 180
E) between 120 and 180
A) 90
B) 109
C) 120
D) 180
E) between 120 and 180

Unlock Deck
Unlock for access to all 108 flashcards in this deck.
Unlock Deck
k this deck
62
Predict the ideal bond angles in FNO using the molecular shape given by the VSEPR theory.
A) 90
B) 109
C) 120
D) 180
E) between 120 and 180
A) 90
B) 109
C) 120
D) 180
E) between 120 and 180

Unlock Deck
Unlock for access to all 108 flashcards in this deck.
Unlock Deck
k this deck
63
What is the molecular shape of ClF4¯ as predicted by the VSEPR theory?
A) square pyramidal
B) square planar
C) see-saw
D) octahedral
E) tetrahedral
A) square pyramidal
B) square planar
C) see-saw
D) octahedral
E) tetrahedral

Unlock Deck
Unlock for access to all 108 flashcards in this deck.
Unlock Deck
k this deck
64
Use VSEPR theory to decide which one of the following ions and molecules is likely to be planar. (The central atom is always first in the formula.)
A) BrF3
B) H3O+
C) PCl3
D) SO42¯
E) SF4
A) BrF3
B) H3O+
C) PCl3
D) SO42¯
E) SF4

Unlock Deck
Unlock for access to all 108 flashcards in this deck.
Unlock Deck
k this deck
65
Predict the smallest actual bond angle in BrF3 using the VSEPR theory.
A) more than 120
B) exactly 120
C) between 109 and 120
D) between 90 and 109
E) less than 90
A) more than 120
B) exactly 120
C) between 109 and 120
D) between 90 and 109
E) less than 90

Unlock Deck
Unlock for access to all 108 flashcards in this deck.
Unlock Deck
k this deck
66
Predict the ideal bond angles around carbon in C2I2 using the molecular shape given by the VSEPR theory.
A) 90
B) 109
C) 120
D) 180
E) None of these choices is correct.
A) 90
B) 109
C) 120
D) 180
E) None of these choices is correct.

Unlock Deck
Unlock for access to all 108 flashcards in this deck.
Unlock Deck
k this deck
67
Use VSEPR theory to decide which one of the following molecules and ions will definitely have at least one 90 bond angle in it. (In each case except water, the central atom is the first one in the formula.)
A) AlCl4¯
B) NH3
C) PCl5
D) CO2
E) H2O
A) AlCl4¯
B) NH3
C) PCl5
D) CO2
E) H2O

Unlock Deck
Unlock for access to all 108 flashcards in this deck.
Unlock Deck
k this deck
68
Predict the ideal bond angles in AsCl3 using the molecular shape given by the VSEPR theory.
A) 90
B) 109
C) 120
D) 180
E) between 110 and 120
A) 90
B) 109
C) 120
D) 180
E) between 110 and 120

Unlock Deck
Unlock for access to all 108 flashcards in this deck.
Unlock Deck
k this deck
69
Use VSEPR theory to predict the electron group arrangement around iodine, the central atom in the ion IF2¯.
A) octahedral
B) trigonal bipyramidal
C) tetrahedral
D) trigonal planar
E) bent
A) octahedral
B) trigonal bipyramidal
C) tetrahedral
D) trigonal planar
E) bent

Unlock Deck
Unlock for access to all 108 flashcards in this deck.
Unlock Deck
k this deck
70
What is the molecular shape of SiF62¯ as predicted by the VSEPR theory? 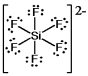
A) trigonal bipyramidal
B) hexagonal
C) tetrahedral
D) see-saw
E) octahedral

A) trigonal bipyramidal
B) hexagonal
C) tetrahedral
D) see-saw
E) octahedral

Unlock Deck
Unlock for access to all 108 flashcards in this deck.
Unlock Deck
k this deck
71
What is the molecular shape of ClF2¯ as predicted by the VSEPR theory?
A) linear
B) bent
C) see-saw
D) T-shaped
E) L-shaped
A) linear
B) bent
C) see-saw
D) T-shaped
E) L-shaped

Unlock Deck
Unlock for access to all 108 flashcards in this deck.
Unlock Deck
k this deck
72
Use VSEPR theory to decide which one of the following molecules and ions will have a trigonal pyramidal geometry. (The central atom is always first in the formula.)
A) PCl3
B) BF3
C) SO3
D) BrF3
E) CO32¯
A) PCl3
B) BF3
C) SO3
D) BrF3
E) CO32¯

Unlock Deck
Unlock for access to all 108 flashcards in this deck.
Unlock Deck
k this deck
73
Predict the ideal bond angles in IF2¯ using the molecular shape given by the VSEPR theory.
A) 60
B) 90
C) 109
D) 120
E) 180
A) 60
B) 90
C) 109
D) 120
E) 180

Unlock Deck
Unlock for access to all 108 flashcards in this deck.
Unlock Deck
k this deck
74
List all possible molecular geometries (shapes) for a nonpolar molecule with the formula AX4.
A) tetrahedral
B) seesaw
C) square planar
D) either tetrahedral or square planar
E) All of these choices are possible.
A) tetrahedral
B) seesaw
C) square planar
D) either tetrahedral or square planar
E) All of these choices are possible.

Unlock Deck
Unlock for access to all 108 flashcards in this deck.
Unlock Deck
k this deck
75
Which one of the following molecules and ions will have a planar geometry?
A) PCl3
B) BF4¯
C) XeF4
D) BrF5
E) H3O+
A) PCl3
B) BF4¯
C) XeF4
D) BrF5
E) H3O+

Unlock Deck
Unlock for access to all 108 flashcards in this deck.
Unlock Deck
k this deck
76
What is the molecular shape of XeO2F2 as predicted by the VSEPR theory? 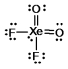
A) square planar
B) tetrahedral
C) square pyramidal
D) see-saw
E) octahedral
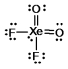
A) square planar
B) tetrahedral
C) square pyramidal
D) see-saw
E) octahedral

Unlock Deck
Unlock for access to all 108 flashcards in this deck.
Unlock Deck
k this deck
77
Predict the ideal bond angles in GeCl4 using the molecular shape given by the VSEPR theory.
A) 90
B) 109
C) 120
D) 180
E) < 90
A) 90
B) 109
C) 120
D) 180
E) < 90

Unlock Deck
Unlock for access to all 108 flashcards in this deck.
Unlock Deck
k this deck
78
What is the molecular shape of SCl3F as predicted by the VSEPR theory?
A) linear
B) bent
C) see-saw
D) T-shaped
E) trigonal pyramidal
A) linear
B) bent
C) see-saw
D) T-shaped
E) trigonal pyramidal

Unlock Deck
Unlock for access to all 108 flashcards in this deck.
Unlock Deck
k this deck
79
Predict the actual bond angles in SF3+ using the VSEPR theory.
A) more than 120
B) exactly 120
C) between 109 and 120
D) between 90 and 109
E) less than 90
A) more than 120
B) exactly 120
C) between 109 and 120
D) between 90 and 109
E) less than 90

Unlock Deck
Unlock for access to all 108 flashcards in this deck.
Unlock Deck
k this deck
80
Predict the actual bond angle in SeCl2 using the VSEPR theory.
A) more than 120
B) between 109 and 120
C) between 90 and 109
D) exactly 90
E) less than 90
A) more than 120
B) between 109 and 120
C) between 90 and 109
D) exactly 90
E) less than 90

Unlock Deck
Unlock for access to all 108 flashcards in this deck.
Unlock Deck
k this deck



